Water steam
| Water vapor | |
|---|---|
| Systematic name | Water vapor |
| Liquid State | Water |
| Solid State | Ice, snow |
| Properties | |
| Liquefaction point | 100 °C |
| Individual gaseous | 461,5 J/(kg·K) |
| Latent evaporation heat | 2.27 MJ/kg |
| Masa molar | 18,02 g/mol |
| Specific heat | 2,01 kJ/(kg·K)
0.48 cal/(g·°C) |
Water vapor is a gas obtained by evaporation or boiling of liquid water or by sublimation of ice. It is odorless and colorless.
Water vapor is responsible for environmental humidity. Under certain conditions, at high concentrations, part of the water that is in the form of vapor condenses constituting drops of liquid water in suspension, thus forming fog or, at greater heights above the ground, clouds.
We can also appreciate the water vapor in our exhalation in cold climates and with high humidity.
Properties
Evaporation
Whenever a water molecule leaves a surface and diffuses into a surrounding gas, it is said to have evaporated. Each individual water molecule that changes between a more associated state (liquid) and a less associated state (vapor/gas) does so by absorbing or releasing kinetic energy. The aggregate measurement of this kinetic energy transfer is defined as thermal energy and occurs only when there is a differential in the temperature of the water molecules. Liquid water that turns into water vapor carries a portion of heat with it, in a process called evaporative cooling. The amount of water vapor in the air determines how often the molecules will return to the surface. When net evaporation occurs, the body of water will experience net cooling directly related to the loss of water.
In the United States, the National Weather Service measures the actual rate of evaporation from a standardized "tray" outdoors, at various locations across the country. Others do the same around the world. Data for the United States is collected and compiled into an annual evaporation map. Measurements range from 30 to over 120 inches per year. Formulas can be used to calculate the evaporation rate from a water surface such as a swimming pool. In some countries, the evaporation rate far exceeds the precipitation rate.
Evaporative cooling is restricted by atmospheric conditions. Humidity is the amount of water vapor in the air. The vapor content of the air is measured with devices known as hygrometers. Measurements are generally expressed as specific humidity or percent relative humidity. The temperatures of the atmosphere and the surface of the water determine the equilibrium vapor pressure; 100% relative humidity occurs when the partial pressure of water vapor is equal to the equilibrium vapor pressure. This condition is often known as full saturation. Humidity ranges from 0 grams per cubic meter in dry air to 30 grams per cubic meter when steam is saturated at 30 °C.
Sublimation
Sublimation is the process by which water molecules directly leave the ice surface without first becoming liquid water. Sublimation explains the slow disappearance in midwinter of ice and snow at temperatures too low to cause melting. Antarctica shows this effect to a unique degree because it is by far the continent with the lowest rate of precipitation on Earth. As a result, there are large areas where millennia-old layers of snow have sublimated, leaving behind the non-volatile materials they contained. This is extremely valuable for certain scientific disciplines, a dramatic example is the collection of meteorites that are exposed in unparalleled quantities and in excellent states of preservation.
Sublimation is important in the preparation of certain classes of biological samples for scanning electron microscopy. Specimens are typically prepared by cryofixation and freeze-fracture, after which the broken surface is lyophilized and abraded by exposure to vacuum until it shows the required level of detail. This technique can display protein molecules, organelle structures, and lipid bilayers with very low degrees of distortion.
Condensation
Water vapor will only condense on another surface when that surface is colder than the dew point temperature, or when the equilibrium of water vapor in air has been exceeded. When water vapor condenses on a surface, there is net heating of that surface. The water molecule brings with it heat energy. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog, and precipitation (usually only when facilitated by cloud condensation nuclei). The dew point of a package of air is the temperature to which it must cool before water vapor in the air begins to condense. Condensation in the atmosphere forms cloud droplets.
In addition, net condensation of water vapor occurs on surfaces when the surface temperature is at or below the dew point temperature of the atmosphere. Deposition is a separate phase transition from condensation that leads to the direct formation of ice from water vapor. Frost and snow are examples of deposition.
There are several cooling mechanisms by which condensation occurs: 1) Direct heat loss by conduction or radiation. 2) Cooling by the drop in air pressure that occurs with air elevation, also known as adiabatic cooling. Air can be lifted by mountains, which deflect air upward, by convection, and by warm and cold fronts. 3) Advective cooling: cooling due to the horizontal movement of air.
Chemical Reactions
Several chemical reactions have water as a product. If the reactions take place at temperatures above the dew point of the surrounding air, water will form as vapor and local humidity will increase, if below the dew point local condensation will occur. Typical reactions that result in the formation of water are the burning of hydrogen or hydrocarbons in air or other oxygen-containing gas mixtures, or as a result of reactions with oxidants.
Similarly, other chemical or physical reactions can take place in the presence of water vapor, resulting in the formation of new chemicals, such as oxidation of iron or steel, polymerization (certain polyurethane foams and cyanoacrylate glues cure on exposure to atmospheric moisture) or change of shapes. such as when anhydrous chemicals can absorb enough vapor to form a crystalline structure or alter an existing one, sometimes resulting in characteristic color changes that can be used for measurement.
In the Earth's atmosphere
Fizzy water represents a small but environmentally significant component of the atmosphere. The percentage of water vapor in the air at the surface varies from 0.01% at -42 °C to 4.24% when the dew point is 30 °C. More than 99% of atmospheric water is in the form of vapor, rather than liquid water or ice, and approximately 99.13% of water vapor is contained in the troposphere. The condensation of water vapor to the liquid or ice phase is responsible for clouds, rain, snow, and other precipitation, all of which are among the most important elements of what we experience as weather. Less obvious, the latent heat of vaporization that is released into the atmosphere when condensation occurs, is one of the most important terms in the atmospheric energy balance on both a local and global scale. For example, the release of latent heat in atmospheric convection is directly responsible for driving destructive storms like tropical cyclones and severe thunderstorms. Water vapor is an important greenhouse gas due to the presence of the hydroxyl bond which absorbs strongly in the infrared.
Water vapor is the "working medium" of the atmospheric thermodynamic engine that transforms the thermal energy of solar radiation into mechanical energy in the form of winds. The transformation of thermal energy into mechanical energy requires an upper and lower temperature level, as well as a working medium that goes back and forth between them. The upper level of temperature is given by the ground or the surface of the earth's water, which absorbs the incoming solar radiation and heats, evaporating the water. Moist, warm air on the ground is lighter than its surroundings and rises to the upper limit of the troposphere. There, the water molecules radiate their heat energy into outer space, cooling the surrounding air. The upper atmosphere constitutes the lowest temperature level of the atmospheric thermodynamic engine. Water vapor in the now cold air condenses and falls to the ground as rain or snow. The cold and dry air, now heavier, also descends to the ground; The atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the ground to the upper atmosphere, where water molecules can radiate it into outer space. Due to the rotation of the earth and the resulting Coriolis forces, this vertical atmospheric convection is also converted into horizontal convection, in the form of cyclones and anticyclones, which carry evaporated water over the oceans into the interior of the continents, allowing vegetation grows... the atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the ground to the upper atmosphere, where water molecules can radiate it into outer space. Due to the rotation of the earth and the resulting Coriolis forces, this vertical atmospheric convection is also converted into horizontal convection, in the form of cyclones and anticyclones, which carry evaporated water over the oceans into the interior of the continents, allowing vegetation grows the atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the ground to the upper atmosphere, where water molecules can radiate it into outer space. Due to the rotation of the earth and the resulting Coriolis forces, this vertical atmospheric convection is also converted into horizontal convection, in the form of cyclones and anticyclones, which carry evaporated water over the oceans into the interior of the continents, allowing the vegetation grows.
Water in Earth's atmosphere is not merely below its boiling point (100 °C), but at altitude below its freezing point (0 °C), because water is the highly polar attraction. When combined with its quantity, water vapor has a relevant dew point and freezing point, unlike, say, carbon dioxide and methane. Thus, water vapor has a scale height a fraction of that of the bulk atmosphere, as the water condenses and leaves, mainly in the troposphere, the lowest layer of the atmosphere. Carbon dioxide (CO2) and methane, being well mixed in the atmosphere, tend to rise above water vapor. The absorption and emission of both compounds contribute to the emission of the Earth into space and, therefore, to the planetary greenhouse effect. This greenhouse effect forcing is directly observable, through different spectral characteristics compared to water vapor, and is observed to increase with increasing CO2 level. Conversely, the addition of water vapor at high altitudes has a disproportionate impact, so aircraft traffic it has a disproportionately high warming effect. Methane oxidation is also a major source of water vapor in the stratosphere, adding approximately 15% to methane's global warming effect.
In the absence of other greenhouse gases, Earth's water vapor would condense on the surface; this probably has happened, possibly more than once. Therefore, scientists distinguish between non-condensable (drives) and condensable (drives) greenhouse gases, i.e. the previous water vapor feedback.
Fog and clouds form through condensation around cloud condensation nuclei. In the absence of nuclei, condensation will only occur at much lower temperatures. Under conditions of persistent condensation or deposition, cloud droplets or snowflakes form, precipitating when they reach a critical mass.
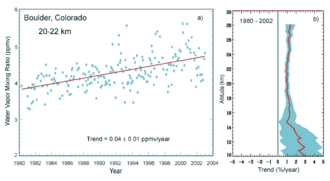
Atmospheric water vapor concentration varies greatly between locations and hours, from 10 ppmv in the coldest air to 5% (50,000 ppmv) in humid tropical air, and can be measured with a combination of ground-based observations, weather balloons, and satellites. The water content of the atmosphere as a whole is constantly being depleted by precipitation. At the same time, it is constantly being replenished by evaporation, primarily from oceans, lakes, rivers, and moist land. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, plant transpiration, and various other biological and geological processes. At any given time there is about 1.29 x 10 16 liters of water in the atmosphere. The atmosphere contains 1 part in 2,500 of the fresh water and 1 part in 100,000 of the total water on Earth. The average global water vapor content in the atmosphere is approximately sufficient to cover the planet's surface with a layer of liquid water. about 25 mm deep. The average annual precipitation for the planet is about 1 meter, a comparison that implies a rapid renewal of water in the air; on average, the residence time of a water molecule in the troposphere is approximately 9 to 10 days.
Global mean water vapor is about 0.25% of the atmosphere by mass and also varies seasonally, in terms of contribution to atmospheric pressure between 2.62 hPa in July and 2.33 hPa in December. IPCC AR6 expresses medium confidence in the increase in total water vapor at around 1-2% per decade; it is expected to increase by around 7% per °C warming.
Episodes of surface geothermal activity, such as volcanic eruptions and geysers, release varying amounts of water vapor into the atmosphere. Such eruptions can be large in human terms, and large explosive eruptions can inject exceptionally large masses of water exceptionally high into the atmosphere, but as a percentage of total atmospheric water the role of such processes is trivial. The relative concentrations of the various gases emitted by volcanoes vary considerably by site and by the particular event at any one site. However, water vapor is consistently the most common volcanic gas; as a rule, it comprises more than 60% of the total emissions during a subaerial eruption.
Atmospheric water vapor content is expressed using several measurements. These include vapor pressure, specific humidity, mixing ratio, dew point temperature, and relative humidity.