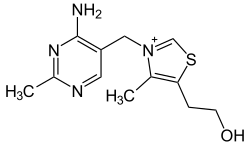
Vitamin B1
Vitamin B1, also known as thiamine (morality vitamin), is a water-soluble, alcohol-insoluble vitamin that forms part of the B complex. Its absorption it occurs in the small intestine (jejunum, ileum) as free thiamine and as thiamine diphosphate (TDP), which is favored by the presence of vitamin C and folic acid, but inhibited by the presence of ethanol (ethyl alcohol). It is necessary in the daily diet of most vertebrates and some microorganisms. Its deficiency in the human organism causes diseases such as beriberi and Korsakoff syndrome. Chemically, it consists of two interconnected organic cyclic structures: a pyrimidine ring with an amino group, and a sulfur-containing thiazole ring linked to the pyrimidine by a methylene bridge.
History of Thiamine
Thiamine was discovered in 1910 by Umetaro Suzuki in Japan while investigating how rice bran cured beriberi patients. He named it aberic acid, thanks to the fact that he observed the effect it had on the symptoms of this disease, but did not determine its chemical composition. It was in 1926 when Jansen and Donath first isolated and crystallized thiamine from rice bran (they named it aneurin, because it was identified as an antineuritic vitamin). Its chemical composition and synthesis was finally reported by Robert R. Williams in 1935. The name thiamine designates the presence of sulfur and an amino group in the complex molecule. This is also converted into a cofactor, called TPP (thiamine pyrophosphate or thiamine pyrophosphate), which participates among others in decarboxylation reactions.
Active forms of thiamine
Thiamine Diphosphate (TDP)
Its active form, thiamine pyrophosphate or thiamine diphosphate, is synthesized by the enzyme thiamine-pyrophosphokinase, which requires free thiamine, magnesium and ATP (adenosine triphosphate) and acts as a coenzyme in the metabolism of carbohydrates. carbon, allowing pyruvic acid or alpha-ketoglutaric acid to be metabolized. In addition, it participates in the synthesis of substances that regulate the nervous system. The following examples include:
In mammals
- As a coenzyme of the dehydrogenase pyruvate (key enzyme in the oxidative descarboxylation of the pyruvate for the formation of acetyl-CoA) and alpha-cetoglutarato dehydrogenasa (enzyme of the Krebs cycle).
- Coenzyme of the dehydrogenase complex of ceto acid alpha from branched chain amino acids. Enzymes that catalyze the separation and transfer of aldehyde groups. Therefore, the TPP acts as a transient conveyor of these aldehyde groups, which join the tiazole ring.
- Coenzyme of the transcetolass for the formation of cetase (via of the pentase to synthesize NADPH and the pentase ribosa and desoxirribosa).
In other species
- Decarboxylase Pyruvate Coenzyme (in yeast)
- In bacteria the coenzyme of Glioxilato Carboligasa, which participates in the formation of Semialdehido tartroncio
Thiamine Triphosphate (TTP)
Thiamine triphosphate or thiamine triphosphate (TTP) participates in decarboxylation reactions, for example in the conversion of pyruvate to Acetyl-CoA, in the conversion of 2-oxoglutarato to succinyl-CoA and in the pentose pathway. transketolase cofactor. In general, it acts as a cofactor in decarboxylation reactions of an α-oxo-acid to aldehyde, such as in alcoholic fermentation (pyruvate decarboxylase); in the generation of α-ketols from α-oxo-acids, such as in the oxidation of pyruvate to Acetyl-CoA or in the synthesis of acetolactate, a precursor of valine and leucine.
Thiamine triphosphate or thiamine triphosphate (TTP) has been considered a specific neuroactive form of thiamine. However, it has been shown to exist in bacteria, fungi, plants, and animals, suggesting that it has a much more general cellular role. It is synthesized from thiamine pyrophosphate (TDP) and ATP through the enzyme TDP-ATP phosphoryltransferase (which is expressed in the brain, kidney, liver, and heart). Its function is associated with the non-coenzymatic function of thiamine, and is related to the synthesis of substances that regulate the nervous system.
Not to be confused with thymidine triphosphate or thymidine triphosphate (TTP), a deoxynucleoside.[citation needed]
Fonts
Vitamin B1 or thiamine is found naturally in: yeast, legumes, whole grains, oats, wheat, corn, nuts, eggs, organ meats (liver, heart, kidney), pork, beef, potatoes, enriched rice, whole rice, sesame seeds, enriched white flour, and yerba mate. Milk and its derivatives, as well as fish, shellfish, are not considered a good source of this vitamin.
Inhibitors
The main inhibitors of vitamin B1 or thiamine are:
Pyrithiamine
Pyrithiamine is an analogue of thiamine. Pyrithiamine has a pyridine ring as its central ring instead of the thiazol ring of Thiamin Pyro Phosphate TPP. Pyrithiamine is an antibiotic from the RiboSwitches family that inhibits growth in fungi and bacteria. This analogue causes symptoms of paralysis. and has been shown to displace thiamine from nerve preparations.
Tetrodotoxin
Tetrodotoxin (TTX) is a neurotoxin, it blocks nerve conduction by inhibiting sodium absorption and also promotes the release of thiamine by neuronal membranes.
Thiaminase
It is an anti-vitamin that breaks the bond that joins the two rings (thiazol-pyrimidine) of thiamine in such a way that it becomes non-functional. Thiaminase is mainly in raw foods, such as freshwater fish, among others, as well as other foods such as tea and coffee. The bacteria of the intestinal flora also synthesize it.
Alcoholic beverages
Ethanol is another great inhibitor, thanks to the fact that it also tends to compete with the vitamin, preventing its absorption.
Nutrition
Thiamine plays an important role in mainly carbohydrate metabolism, so a carbohydrate-rich diet requires more thiamine than a high-fat diet. In addition, thiamine is involved in the metabolism of fats, proteins, and nucleic acids (because is a cofactor in the pentose pathway). It is essential for normal growth and development and helps maintain the proper functioning of the heart, nervous and digestive systems. Thiamine is soluble in water, and the reserve in the body is low; concentrating on skeletal muscle primarily; in the form of TDP (80%) TTP (10%) and the rest as free thiamine.
Studies published in August 2007 indicate that the intake of foods rich in thiamine would prevent certain serious effects of diabetes (especially cardiovascular, renal and ocular complications) since thiamine protects cells against high levels of glucose.
Its lack of consumption causes an abnormality in the metabolism and can cause diarrhea, polyneuritis, cardiac dilation and weight loss, so it must be ingested in adequate quantities to avoid contracting these diseases.
Absorption and deposit
Thiamine is absorbed by a passive mechanism (at high doses) and by an active mechanism (at low doses) and in this process it is phosphorylated, its absorption site is in the small intestine through two specific transporters: the thiamine transporter thiamine type 1 (hTHTR1) and transporter type 2 (hTHTR2), although other less metabolically important transporters may also be involved.
Once absorbed, it circulates bound to albumin and erythrocytes. It is mainly deposited in the form of thiamine pyrophosphate, its most important storage site being muscle, but also in the heart, liver, kidneys, and brain.
The body store reaches 30 mg and its biological half-life is 9 to 18 days. It was the first molecule to be discovered with the characteristics of vitamins, and since it was chemically an amine, it was called "amine vitae" (amine of life), from which it came to be called vitamin. It is necessary to disintegrate carbohydrates and to be able to take advantage of their energetic principles.
Thiamine deficiency
Most dietary thiamine deficiencies are due to insufficient intake. Thiamine deficiency can be caused by malnutrition, alcoholism or a diet rich in foods that are a source of thiaminase (antithiamine factor, present in raw freshwater fish, raw crustaceans, and in beverages such as tea, coffee).
Well-known syndromes of severe thiamine deficiency include beriberi and Wernicke-Korsakoff syndrome (cerebral beriberi), diseases also common in chronic alcoholism.
Thiamine deficiency can be evidenced by giving the patient intravenous glucose.
Other not very severe deficiencies include behavioral problems at the nervous system level, irritability, depression, lack of memory and ability to concentrate, lack of mental dexterity, palpitations at the cardiovascular level, hypertrophy of the heart.
It has also been thought that many people with diabetes are deficient in thiamine and that this may be linked to complications of the disease.
Diagnostic tests for thiamine or B1 deficiency
One of the tests that diagnoses thiamine deficiency consists of measuring the activity of transketolases in erythrocytes erythrocyte transketolase, the enzymatic activity is normal in mild deficiency, but increases with the addition of the coenzyme (TPP). Another is to directly measure thiamine in blood, following the conversion of thiamine to a fluorescent thiochrome derivative.
Recommended Daily Intake
(Dose in Milligrams per day)
| Age | Men | Women |
|---|---|---|
| 1-3 Years | 0.5 | 0.5 |
| 4-8 Years | 0.6 | 0.6 |
| 8-13 | 0.9 | 0.9 |
| 14-18 Years | 1.21 | 1.21 |
| + 18 Years | 1.52 | 1.52 |
| Pregnant or Infant | 1.4 |
References and bibliography
- ↑ CAS number
- ↑ a bc J., Marshall, William; Marta, Lapsley, (2013). "20." Clinical biochemistry (7th ed edition). Elsevier. p. 326. ISBN 9788490221150. OCLC 846784551.
- ↑ E., Metzler, David (©2001-2003). "14." Biochemistry: the chemical reactions of living cells (2nd ed edition). Harcourt/Academic Press. p. 734-7836. ISBN 9780124925410. OCLC 44852069.
- ↑ History of thiamine contained in 6 paragraphs https://masonnatural.pe/
- ↑ a b E., Metzler, David (©2001-2003). "14." Biochemistry: the chemical reactions of living cells (2nd ed edition). Harcourt/Academic Press. p. 736. ISBN 9780124925410. OCLC 44852069.
- ↑ E., Metzler, David (©2001-2003). Biochemistry: the chemical reactions of living cells (2nd ed edition). Harcourt/Academic Press. ISBN 9780124925410. OCLC 44852069.
- ↑ He., Zhang, Li; Zhen., Xi, Jyoti., Chattopadhyaya, service), Wiley InterScience (Online (2011). Medicinal chemistry of nucleic acids. John Wiley & Sons. ISBN 9781118092811. OCLC 746324268.
- ↑ He., Zhang, Li; Zhen., Xi, Jyoti., Chattopadhyaya, service), Wiley InterScience (Online (2011). "4." Medicinal chemistry of nucleic acids. John Wiley & Sons. ISBN 9781118092811. OCLC 746324268.
- ↑ E., Metzler, David (©2001-2003). Biochemistry: the chemical reactions of living cells (2nd ed edition). Harcourt/Academic Press. p. 736. ISBN 9780124925410. OCLC 44852069.
- ↑ E., Metzler, David (©2001-2003). "14." Biochemistry: the chemical reactions of living cells (in 736-737) (2nd ed edition). Harcourt/Academic Press. ISBN 9780124925410. OCLC 44852069.
- ↑ Gutierrez, Jean L.; Kerns, Jennifer C. (1 March 2017). "Thiamin." Advances in Nutrition (in English) 8 (2): 395-397. ISSN 2161-8313. doi:10.3945/an.116.013979. Consultation on March 29, 2019.
- ↑ a b J., Marshall, William; Marta, Lapsley, (2013). Clinical biochemistry (7th ed edition). Elsevier. ISBN 9788490221150. OCLC 846784551.
Contenido relacionado
Glaucoma
Come to
Transverse muscle of the nose