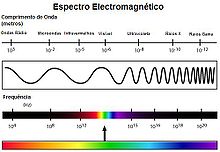
Ultraviolet radiation
The term ultraviolet radiation or UV radiation refers to electromagnetic radiation whose wavelength is approximately between 100 nm (100×10−9 m) and 400 nm (400×10−9 m). Its name comes from the fact that its range starts from shorter wavelengths than what the human eye identifies as violet light, but said light or wavelength is invisible to the human eye, being above the visible spectrum. This radiation is an integral part of the sun's rays and produces several health effects as it is between non-ionizing and ionizing radiation.
Discovery
The discovery of ultraviolet radiation is associated with the experimentation of the darkening of silver salts when exposed to sunlight. In 1801 the German physicist Johann Wilhelm Ritter discovered that invisible rays located just behind the violet end of the visible spectrum were especially effective in darkening paper impregnated with silver chloride. He termed these rays & # 34;deoxidizing rays & # 34; to emphasize their chemical reactivity and to distinguish them from "heat rays" (discovered by William Herschel) that were on the other side of the visible spectrum. Shortly thereafter the term "chemical rays" was adopted. These two terms remained quite popular throughout the 19th century. Finally these terms were giving way to the more modern infrared and ultraviolet radiation respectively.
Visibility
Ultraviolet rays are invisible to most humans. The lens of the human eye blocks most of the radiation in the 300-400 nm (nanometer) wavelength range; shorter wavelengths are blocked by the cornea. Humans also lack color receptor adaptations for ultraviolet rays. However, the photoreceptors in the retina are sensitive to near-ultraviolet rays, and people who lack a lens (a condition known as aphakia) perceive near-ultraviolet rays as whitish-blue or whitish-violet. In some conditions, children and young adults can see ultraviolet down to wavelengths around 310 nm. Near-ultraviolet radiation is visible to insects, some mammals, and birds. Small birds have a fourth color receptor for ultraviolet rays; this gives the birds "true" UV vision.
Subtypes
According to their wavelength, several subtypes of ultraviolet rays are distinguished:
| Name | Abbreviation | Wave length (nm) | Energy by photon (eV) | Notes/Alternative Names |
|---|---|---|---|---|
| Ultraviolet A (long wave) | UVA | 400-315 | 3.10-3.94 | Long-wave UV, black light, not absorbed by the ozone layer: soft UV. |
| Ultraviolet B (average wave) | UVB | 315-280 | 3,94-4,43 | Medium-wave UV, mainly absorbed by the ozone layer: intermediate UV; Dorno radiation. |
| Ultraviolet C (short end) | UVC | 280-100 | 4,43-12,40 | Shortwave UV, germicide UV, ionizing radiation in shorter wavelengths, completely absorbed by the ozone layer and the atmosphere: hard UV. |
| Near (near) | UNOV | 400-300 | 3.10-4.13 | Visible for birds, insects and fish. |
| Medium ultraviolet (middle) | MUV | 300-200 | 4,13-6,20 | |
| Far ultraviolet (far) | FUV | 200-122 | 6,20-10,16 | ionizing radiation in shorter wavelengths. |
| Lyman-alpha line | H Lyman-α / Ly-α | 122-121 | 10,16-10,25 | Spectral line at 121.6 nm, 10.20 eV. |
| Vacuum Ultraviolet | VUV | 200-10 | 6,20-124 | Very absorbed by atmospheric oxygen, although wavelengths of 150 to 200 nm can spread through nitrogen. |
| Extreme ultraviolet | EUV | 121-10 | 10,25-124 | Fully ionizing radiation according to some definitions; completely absorbed by the atmosphere. |
Various solid-state and vacuum devices have been explored for use in different parts of the UV spectrum. Many approaches try to adapt sensing devices to visible light, but these can suffer from unwanted response to visible light and various instabilities. Ultraviolet can be detected by suitable photodiodes and photocathodes, which can be tailored to be sensitive to different parts of the UV spectrum. There are photomultipliers sensitive to UV rays. Spectrometers and radiometers are manufactured to measure UV radiation. Silicon detectors are used across the spectrum.
Vacuum ultraviolet, or VUV, wavelengths (less than 200 nm) are strongly absorbed by molecular oxygen in the air, although longer wavelengths, around 150-200 nm, can propagate at through nitrogen. Therefore, scientific instruments can utilize this spectral range by operating in an oxygen-free atmosphere (commonly pure nitrogen), without the need for expensive vacuum chambers. Some significant examples are 193 nm photolithography equipment, for manufacturing integrated circuits, and circular dichroism spectrometers.
The technology for VUV instrumentation was largely driven by solar astronomy for many decades. Although optics can be used to eliminate unwanted visible light contaminating the UVV, in general; detectors may be limited by their response to non-VUV radiation, and the development of 'sun-blind' devices has been an important area of research. Wide-range solid-state devices or vacuum devices with high-cutoff photocathodes may be attractive compared to silicon diodes.
Extreme ultraviolet (EUV or sometimes XUV) is characterized by a transition in the physics of interaction with matter. Wavelengths longer than about 30 nm primarily interact with the outer valence electrons of atoms, while shorter wavelengths primarily interact with inner shell electrons and nuclei. The long end of the EUV spectrum is marked by a prominent He+ spectral line at 30.4 nm. Most known materials absorb EUV, but it is possible to synthesize multi-coated optics that reflect up to 50% of normal incidence EUV radiation. This technology was pioneered on the NIXT and MSSTA sounding rockets in the 1990s, and has been used to make telescopes for solar imaging.

Some sources use the distinction of “hard UV” and “soft UV” (in the case of astrophysics, the limit may be at the Lyman limit, i.e. wavelength 91.2 nm, the "Hard UV" more energetic). The same terms can also be used in other fields, such as cosmetology, optoelectronics, etc. The numerical value of the hard/soft boundary, even within similar scientific fields, does not necessarily coincide; for example, one applied physics journal used a 190 nm boundary between the hard and soft UV regions.
Solar ultraviolet
Very hot objects emit ultraviolet radiation (see blackbody radiation). The Sun emits ultraviolet radiation at all wavelengths, including extreme ultraviolet where it intersects X-rays at 10 nm. Extremely hot stars emit proportionally more ultraviolet radiation than the Sun. Sunlight in space at the top of Earth's atmosphere (see solar constant) is made up of about 50% infrared light, 40% solar visible and 10% UV light, for a total intensity of about 1400 W/m² in a vacuum.
The atmosphere blocks about 77% of the Sun's UV rays when the Sun is highest in the sky (at the zenith), and absorption increases at shorter UV wavelengths. At ground level, with the sun at the zenith, sunlight is 44% visible light, 3% ultraviolet, and the rest infrared. Of the ultraviolet radiation that reaches the Earth's surface, most 95% are the longer wavelengths of UVA, and the small remainder UVB. Almost no UVC reaches the Earth's surface. The fraction of UVB that remains in UV radiation after passing through the atmosphere is highly dependent on cloudiness and atmospheric conditions. On "partly cloudy" days, the patches of blue sky that show up between the clouds are also sources of (scattered) UVA and UVB, which are produced by Rayleigh scattering in the same way as visible blue light from those parts of the sky. darling. UVB also plays an important role in plant development, as it affects most plant hormones. During total clouding, the amount of absorption due to clouds is highly dependent on cloud thickness and latitude, with no clear measurements correlating specific thickness and UVB absorption.
The shorter bands of UVC, as well as the even more energetic UV radiation produced by the Sun, are absorbed by oxygen and generate ozone in the ozone layer when individual oxygen atoms produced by UV photolysis of dioxygen they react with more dioxygen. The ozone layer is especially important in blocking most of the UVB and the remaining part of the UVC that is not blocked by ordinary oxygen in the air.
Blockers, absorbents and windows
Ultraviolet absorbers are molecules used in organic materials (polymers, paints, etc.) to absorb UV radiation and reduce UV degradation (photo-oxidation) of a material. Absorbents can themselves degrade over time, so it is necessary to control absorption levels in aged materials.
In sunscreens, UVA/UVB-absorbing ingredients, such as avobenzone, oxybenzone, and octyl methoxycinnamate, are organic chemical absorbers, or "blockers." They are opposed to inorganic absorbers/'blockers' of UV radiation, such as carbon black, titanium dioxide and zinc oxide.
In the case of clothing, the ultraviolet protection factor (UPF) represents the relationship between the UV rays that cause sunburn without and with the protection of the fabric, similar to the sun protection factor (SPF) of Sunscreens. Standard summer fabrics have UPFs around 6, which means around 20% of UV rays will pass through them.[citation needed]
Nanoparticles suspended in the stained glass windows prevent UV rays from causing chemical reactions that change the colors of the images. A stained glass color reference chipset is planned to be used to calibrate ESA's 2019 ESA color cameras as they will not be affected by the high UV radiation present on the surface of Mars.[ citation required]
Ordinary soda lime glass, such as window glass, is partially transparent to UVA rays, but opaque to the shorter wavelengths, passing about 90% of light above 350 nm, but blocking more 90% of light below 300nm. One study found that car windows let through 3-4% of ambient UV rays, especially if UV rays are higher than 380nm. Other types of car windows can reduce the transmission of UV rays above 335 nm. Fused quartz, depending on its quality, can be transparent even at vacuum UV wavelengths. Crystalline quartz and some crystals such as CaF2 and MgF2 transmit well up to 150 nm or 160 nm wavelengths.
Wood's glass is a deep purplish-blue barium sodium silicate glass with approximately 9% nickel oxide developed during World War I to block visible light for covert communications. It enables daytime infrared and nighttime ultraviolet communications by being transparent between 320nm and 400nm and also barely visible red and longer infrared wavelengths. Its maximum ultraviolet transmission is at 365 nm, one of the wavelengths of mercury lamps.
Artificial sources
“Black Lights”
A blacklight lamp emits long-wavelength UV-A radiation and little visible light. Blacklight fluorescent lamps work similar to other fluorescent lamps, but use a phosphor on the surface of the inner tube that emits UV-A radiation instead of visible light. Some lamps use a deep blue Wood's glass optical filter that blocks almost all visible light with wavelengths greater than 400 nanometers. Others use smooth glass instead of the more expensive Wood's glass, so they appear colored light blue in sight when working. Incandescent blacklights are also produced, which use a filter coating on the casing of an incandescent bulb that absorbs visible light (see next section). They are cheaper, but very ineffective, as they only emit a small fraction of their power as UV. Mercury Vapor blacklights in powers up to 1kW with UV-emitting phosphor and a glass envelope from Wood are used for stage performances and concerts. Blacklights are used in applications where extraneous visible light must be minimized; primarily to observe fluorescence, the colored glow that many substances emit when exposed to UV light. UV-A/UV-B emitting bulbs are also sold for other special purposes such as tanning lamp and reptile breeding.
Shortwave ultraviolet lamps
Shortwave UV lamps are made with a fluorescent lamp tube without a phosphor coating, composed of fused quartz or vycor, since ordinary glass absorbs UV-C. These lamps emit ultraviolet light with two peaks in the UV-C band at 253.7 nm (nanometers) and 185 nm due to the mercury inside the lamp, as well as some visible light. Between 85% and 90% of the ultraviolet light produced by these lamps is at 253.7 nm, while only 5-10% is at 185 nm.[citation required] The fused quartz tube passes the 253.7 nm radiation but blocks the 185 nm wavelength. These tubes have two to three times the UV-C power of a normal fluorescent lamp tube. These low pressure lamps have a typical efficiency of approximately 30-40%, which means that for every 100W (watts) of electricity consumed by the lamp, they will produce approximately 30-40W of total UV output. They also emit bluish-white visible light, due to the other spectral lines of mercury. These "germicidal" lamps are widely used for surface disinfection in laboratories and food industries, as well as for disinfection of water supplies.
Incandescent lamps
Incandescent "blacklight" lamps are also made from an incandescent bulb with a filter coating that absorbs most of the visible light. Halogen lamps with fused quartz envelopes are used as inexpensive UV light sources in the near-UV range, 400-300 nm, in some scientific instruments. Due to its black body spectrum, a filament bulb is a very inefficient ultraviolet source, emitting only a fraction of its energy as UV.
Gas discharge lamps
Specialized UV gas discharge lamps containing different gases produce UV radiation at particular spectral lines for scientific purposes. Argon arc lamps and deuterium arc lamps are often used as stable sources, either windowless or with multiple windows such as magnesium fluoride. They are often the emitting sources in UV spectroscopy equipment for chemical analysis.
Other UV sources with more continuous emission spectra include xenon arc lamps (commonly used as sunlight simulators), deuterium arc lamp, precision xenon arc lamp, and metal halide arc lamp.
The excimer lamp, a UV source developed in the early 2000s, is increasingly used in scientific fields. It has the advantages of high intensity, high efficiency, and operation in a variety of wavelength bands up to vacuum ultraviolet.
Ultraviolet LEDs
Light-emitting diodes (LEDs) can be made to emit radiation in the ultraviolet range. In 2019, following significant advances in the previous five years, 365nm and longer wavelength UV-A LEDs are now available, with efficiencies of 50% at 1.0W power. The most common types of UV LEDs that can be found/purchased today are at 395nm and 365nm wavelengths, both in the UV-A spectrum. When referring to the wavelength of UV LEDs, the nominal wavelength is the maximum wavelength that the LEDs emit, and light at higher and lower wavelength frequencies near the wavelength. wavemax are present, which is important to consider when seeking to apply them for certain purposes.
The cheaper and more common 395 nm UV LEDs are much closer to the visible spectrum, and the LEDs not only operate at their full wavelength, but also emit a purple color, and they end up not emitting pure UV light, unlike other UV LEDs that are deeper in the spectrum. These types of LEDs are increasingly being used in applications such as UV curing, charging glowing objects in the dark, like pictures or toys, and are becoming very popular in a process known as retro-brightening, which speeds up the reconditioning/bleaching process of old plastics and portable flashlights to detect counterfeit money and bodily fluids, and are already successful in digital printing applications and in inert UV curing environments. Power densities approaching 3 W/cm² (30 kW/m²) are now possible, and this, together with recent developments in photoinitiator and resin formulators, makes expansion of LED-cured UV materials likely..
UV-C LEDs are developing rapidly, but may require testing to verify disinfection efficacy. Citations for disinfection of large areas are for non-LED UV sources known as germicidal lamps. They are also used as line sources to replace deuterium lamps in liquid chromatography instruments.
Ultraviolet laser
Gas lasers, laser diodes, and solid-state lasers can all be made to emit ultraviolet rays, and lasers exist that cover the entire UV range. The nitrogen gas laser uses the electronic excitation of nitrogen molecules to emit a beam that is mostly UV. The strongest ultraviolet lines are at 337.1 nm and 357.6 nm in wavelength. Another type of high power gas lasers are excimer lasers. They are widely used lasers that emit in the ultraviolet and vacuum ultraviolet wavelength ranges. Argon fluoride UV excimer lasers operating at 193 nm are now routinely used in the production of integrated circuits by photolithography. The current limit of coherent UV production wavelength is about 126 nm, characteristic of the Ar2* excimer laser.
Direct UV-emitting laser diodes exist at 375 nm. UV diode-pumped solid-state lasers have been demonstrated using crystals of cerium-doped strontium aluminum fluoride (Ce:LiSAF), a process developed in the decade 1990 at Lawrence Livermore National Laboratory. Wavelengths less than 325 nm are generated commercially in diode-pumped solid-state lasers. Ultraviolet lasers can also be made by applying frequency conversion to low frequency lasers.
Ultraviolet lasers have applications in industry (laser engraving), medicine (dermatology and keratectomy), chemistry (Maldi), secure airborne communications, computing (optical storage), and integrated circuit manufacturing.
Tunable Vacuum Ultraviolet (VUV)
The vacuum ultraviolet band (V-UV) (100-200 nm) can be generated by non-linear mixing of 4 waves in gases by sum or difference of frequencies of 2 or more lasers of longer wavelength. Generation is usually done in gases (for example, krypton, hydrogen which are two-photon resonants near 193 nm) or metal vapors (for example, magnesium). By making one of the lasers tunable, the V-UV can be tuned. If one of the lasers is resonant with a transition in the gas or vapor, the production of V-UV is intensified. However, resonances also generate wavelength dispersion, so phase matching can limit the tunable range of the 4-wave mix. Differential frequency mixing (i.e. f1 + f2 − f3) as an advantage over summing frequency mixing because phase matching can provide better tuning.
Extreme UV synchrotron and plasma sources
Lasers have been used to indirectly generate non-coherent extreme ultraviolet (E-UV) radiation at 13.5 nm for extreme ultraviolet lithography. E-UV is not emitted by the laser, but by electron transitions in an extremely hot tin or xenon plasma, which is excited by an excimer laser. This technique does not require a synchrotron, although it can produce UV at the edge of the X-ray spectrum. Synchrotron light sources can also produce all wavelengths of UV light, including those at the borderline of the UV and X-ray spectra, down to 10 nm.
Applications
Ultraviolet light has numerous practical applications. It is used in food and water sterilization, in industrial arc welding, for photochemical curing of inks, paints, plastics, for diagnostic and therapeutic medical treatments, such as UV lamps used in dermatology and cosmetic tanning.
Sterilization
One of the applications of ultraviolet rays is as a form of sterilization. Ultraviolet radiation of certain wavelengths damages the DNA of many microorganisms and prevents them from reproducing. In this way they can eliminate bacteria, viruses and fungi without leaving residues, unlike chemical products. UV sterilization of milk is being studied as an alternative to pasteurization.
Fluorescent lamps
Fluorescent lamps produce UV radiation by ionizing mercury gas at low pressure. A phosphorescent coating on the inside of the tubes absorbs UV radiation and converts it into visible light. Part of the wavelengths emitted by mercury gas are in the UVC range. Unprotected exposure of the skin and eyes to mercury lamps that do not have a conversion phosphor is extremely dangerous.
The light obtained from a mercury lamp is composed mainly of discrete wavelengths. Other practical sources of more continuous spectrum UV radiation include xenon lamps, deuterium lamps, mercury-xenon lamps, metal halide lamps, and the halogen lamp.
There are also fluorescent lamps capable of emitting ultraviolet light or "black light." These lamps use only one type of phosphor instead of the many used in normal fluorescent lamps, and instead of clear glass, a blue-violet color called Wood's glass is used. Wood's glass contains nickel oxide, and blocks almost all visible light above 400nm. The phosphor normally used for an emission spectrum from 368 nm to 371 nm can be either a mixture of europium and strontium fluoroborate (SrB4O7F:Eu2+), or a mixture of europium and strontium borate (SrB4O7:Eu2+), while the phosphor used for the 350 to 353 nm range is lead associated with barium silicate (BaSi2O5:Pb+).
Science and Engineering
Ultraviolet radiation, when illuminating certain materials, becomes visible due to the phenomenon called fluorescence. This method is commonly used to authenticate antiquities and banknotes, as it is a non-invasive and non-destructive examination method. In metallic structures, fluorescent liquids are usually applied to later illuminate it with a black light, and thus detect cracks and other defects.
In forensic science, black light is used to detect traces of blood, urine, semen, and saliva (among others), causing these fluids to fluoresce, making them easier to detect.
UV/VIS (ultraviolet and visible light) spectrophotometry is widely used in analytical chemistry. Lasers such as excimer and nitrogen lasers (TEA) radiate at short wavelengths, with enough energy to atomize samples and obtain atomic emission spectra.
Pest control
Ultraviolet fly traps are used to eliminate small flying insects. Said creatures are attracted to the UV light and then either killed by an electric shock or trapped after touching the trap.
Solar UV rays on Earth
Most of the ultraviolet radiation that reaches Earth from the Sun is in the forms UV-A, UV-B, and UV-C; UV-C radiation does not reach the surface as it is absorbed by oxygen and ozone in the atmosphere; UV-B radiation is partially absorbed by ozone and only reaches the earth's surface in a minimal percentage, so the radiation that arrives is mainly UV-A.
Effects on living beings
UV radiation is highly mutagenic, that is, it induces mutations. In DNA, it causes damage by forming pyrimidine dimers (generally thymine dimers) that shorten the normal length of the bond, generating a deformation of the chain.
The types of UV radiation (A, B and C) are related to the damage they produce in humans: UV-C radiation is the most harmful to life.
UV radiation reaching the earth's surface is largely made up of UV-A radiation (95%) and to a lesser extent UV-B radiation (5%)
Moderate absorption of UV-B ultraviolet rays allows the synthesis of vitamin D in the skin, necessary for the absorption of calcium and its deposition in the bones.
Damages that UV rays can cause to humans include effects on the skin such as irritation, wrinkles, loss of elasticity, blemishes, and cancer. Also possible conditions at the ocular level and can trigger systemic lupus erythematosus or porokeratosis.
In moderate amounts it can activate skin cells called melanocytes in some people, producing a pigmentation known as tanning. Melanocytes have the mission of protecting the body from excesses in solar radiation. But in excessive amounts they are responsible for the typical burns caused by solar radiation.
UV Index
The UV index is an indicator of the intensity of UV radiation from the Sun at the Earth's surface. The UV index also indicates the ability of solar UV radiation to cause skin damage.
Because the index and its representation varied depending on the location, the World Health Organization together with the World Meteorological Organization, the United Nations Environment Program and the International Commission on Non-Ionizing Radiation Protection publish a standard UV index measurement system and a way to present it to the public including an associated color code.
The code can be seen in the following table:
| Color | Risk | UV |
|---|---|---|
| . Green | Low | 2 |
| . Yellow | Moderate | 3-5 |
| . Orange | High | 6-7 |
| . Red | Very high. | 8-10 |
| . Violeta | Extremely high | ▪ 11 |
Ultraviolet vision
Humans, like most mammals, are unable to identify the color of ultraviolet. This may be because their Cretaceous ancestors were primarily nocturnal in order to remain unnoticed and escape predatory dinosaurs. That pattern caused these ancestors to lose their ultraviolet and red photoreceptors. Formerly they had possessed the four different photoreceptors, as is typical of fish, amphibians and reptiles and even birds. With the course of evolution and the massive extinction of the dinosaurs, mammals began to colonize the planet and modified their behavior patterns. They became diurnal, and some orders, such as primates, recovered the red photoreceptor, making it easier to detect ripe fruit. Other orders, such as carnivores and many rodents, retained or recovered the ultraviolet photoreceptor, which is of vital importance. to mark territory as urine and feces effectively reflect ultraviolet light.
In the case of fish, ultraviolet communication, especially in the case of osteichthyans, is of vital importance to escape from a predator that cannot see it.
Electric welding
The photovoltaic arc of electric welding is a source of ultraviolet radiation. This poses a significant risk to the health of welders.
Contenido relacionado
Donna Haraway
Seawater energy
Lyman Spitzer Jr.