Tubulin
The name tubulin refers to a family of 55 Kdalton globular proteins. The tubulin family is made up of alpha (α), beta (β) and gamma (γ) tubulins, which share 35-40% amino acid chain identity, although their similarity to any other known protein is minimal..α- and β-tubulins are the essential subunits of microtubules, while γ-tubulin is a fundamental component of the centrosome. There are also other minor variants, which are not present in all eukaryotic organisms, called delta-tubulin (δ), -epsilon (ε) and -zeta (ζ).
Commonly, tubulin is called a heterodimer made up of two subunits (α and β) which, when assembled in a highly organized manner, generates one of the main components of the cytoskeleton, the microtubules. All eukaryotic cells have microtubules, indicating that the subunits that make them up probably originated when eukaryotes first appeared, approximately 1.5 billion years ago. It is likely that other microtubule-associated proteins also date to eukaryotic origin, such as some members of the kinesin and dynein families, although others are of more recent origin, such as the neuron-specific protein tau.
Conservation of α and β tubulins
Since the tubulin family proteins have a very ancient origin, their sequences might be expected to be highly divergent. However, this is only true for the C-terminus of α and β tubulins. The N-terminal fragments are remarkably conserved with minimal variations. This high degree of conservation is likely imposed by structural constraints on microtubule (MT) assembly and disassembly, together with constraints imposed by the association of proteins such as kinesins and dyneins. Individual members of the tubulin family of different phylogenetic orders have evolved exceptionally slowly, at a rate comparable to that of histones or actin. The high rate of conservation within the tubulin family means that the functional properties of these proteins impose enormous limitations on any sequence diversification, such that mutations can only be accommodated at a few positions without producing a deleterious effect. On the other hand, a conserved modification is likely to be functionally advantageous, and thus surely related to specific properties of tubulins in different orders.
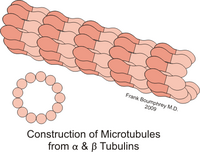
Tubulin and microtubule structure
Protein-protein interactions between microtubule subunits constitute a constraint to the tertiary structure of α and β tubulins. Traditional electron microscopy studies using glutaraldehyde-fixed cells established that a MT is normally composed of 13 aligned tubulin protofilaments. Although variants of this structure have been identified, the number of protofilaments in vivo appears to vary between 12 and 15. The protofilament consists of alternating globular units, with dimensions approximately 50 x 50 x 40 Å, with a repeating unit along the long axis of the protofilament of about 80 Å.
These dimensions are consistent with a 50 kD tubulin monomer forming α/β heterodimers, constituting the 80 Å unit.
Since protofilaments form the basis of MT assembly, longitudinal interactions between heterodimers are likely to be more stable than lateral, inter-protofilament interactions.
Heterodimers assemble into protofilaments in such a way that β-tubulin from one dimer contacts α-tubulin from the next dimer. Therefore, MTs are inherently polar, as they have α-tubulin at one end of the polymer (the 'minus' end) and β-tubulin at the other (the 'plus' end). 3. 4;). The 13 protofilaments that make up an MT are arranged side by side, so that if one follows the α or β subunits laterally around the MT, they are seen to form a three-subunit helix. This means that the helix travels 3 subunits to complete one turn. This type of helix is not perfectly symmetrical, resulting in a "joint" on the MT wall at the place where each helix completes one turn. Protofilaments interact with each other laterally mainly through α–α and β–β contacts, although at the junction α-tubulin contacts β-tubulin.
The tubulin dimer binds 2 moles/mole of the guanosine nucleotide: one is at an exchangeable site, while the second is non-exchangeable. Tubulin purified from neurons contains 1 mol/mol of both GTP and GDP, with GDP being bound to the exchangeable site. The exchangeable site is located on the β subunit, and the non-exchangeable GTP appears to be bound to the α subunit. Assembly of MTs is, under almost all conditions, dependent on GTP or a nonhydrolyzable GTP analogue bound to the α subunit. interchangeable site. This GTP molecule is subsequently hydrolyzed to GDP, which remains bound to the assembled MT and is only exchangeable when the MT is disassembled. This change in interchangeability may be due to packing of the subunits into the assembled MT or an assembly-dependent conformational change.
In vitro, MTs polymerize spontaneously from high concentrations of α and β tubulin, in the presence of GTP and Mg2+. The polymerization process occurs in two steps: 'nucleation', which is the limiting step, followed by rapid elongation. The nucleation step is thought to involve the formation of a pair of short protofilaments, which they would consist of 7, 12, or 18 α/β tubulin dimers. Once this nucleus has formed, it grows rapidly laterally and longitudinally as a sheet, until around 1000 dimers have been assembled; at that moment the sheet closes on itself to form a cylinder. Laminae can also be seen at the growing ends of preformed MTs, suggesting that the MTs are two-dimensional polymers, and not a helical polymer, in their mode of elongation. It is assumed that the MTs assemble in the same way in in vivo, although the concentration of α/β-tubulin inside cells is below the level necessary for the spontaneous nucleation observed in vitro to occur, so the process is catalyzed by MT organizing centers (COMTs). MTOCs), such as centrosomes in animal cells and the spindle pole body in yeast. The need for MTOCs in vivo allows the cell to control when and where the nucleation of MTs occurs. A large body of evidence (genetic experiments, antibody inhibition studies, in vitro complementation assays, and fluorescence and electron microscopy) implicates γ-tubulin as the key protein responsible for the nucleation of MTs in alive.
Types
The "superfamily" Tubulin contains six families: alpha (α), beta (β), gamma (γ), delta (δ), epsilon (ε), and zeta (ζ).
Alpha Tubulin
Subtypes of human α-tubulin include:
- TUBA1A
- TUBA1B
- TUBA1C
- TUBA3C
- TUBA3D
- TUBA3E
- TUBA4A
- TUBA8
Beta Tubulin
- TUBB
- TUBB1
- TUBB2A
- TUBB2B
- TUBB2C
- TUBB3
- TUBB4
- TUBB4Q
- TUBB6
- TUBB8
Tubulin-γ
This protein is highly conserved, and is approximately 30% identical to α and β tubulins, but it does not assemble into the polymeric structure of MTs. Although its activity is concentrated in MTOCs, most of the γ-tubulin is found in the cytosol. Cytosolic γ-tubulin is found primarily in two complexes: the larger γ-tubulin ring complex (termed γTuRC, the acronym for γ-tubulin ring complex) and the small tubulin-γ complex (called γTuSC, for γ-tubulin small complex), which is a subunit of γTuRC and is analogous to the Tub4 complex of Saccharomyces cerevisiae.
The γTuRC complex was initially isolated from Xenopus eggs and Drosophila cells. It consists of approximately 10–14 γ-tubulin molecules and at least six additional proteins, generating a complex of about 2 MDa. This complex is well conserved, as similar complexes have been identified in mammalian cells. From electron microscopy images, the γTuRC complex has been observed to have a flexible open ring structure, around 25 nm in diameter. Based on its structure, it appears that this complex functions as a template, from which the MTs grow. As cellular MTs normally contain 13 protofilaments, the model proposes that γTuRC contains 13 laterally interacting γ-tubulin units. However, data from several studies suggest that the γTuRC complex assembles from preformed γTuSCs, which contain two copies of γ-tubulin and one copy each of the S. cerevisiae Spc97 and Spc98 homologous proteins. This implies that the γTuRC complex must contain an even number of γ-tubulin, instead of 13 as initially proposed. It has been proposed that the components of the γTuRC that are not part of the γTuSC form a "cap" (cap) that covers the end "minus" of the MTs, which could regulate their activity, confer stability to the helix and/or anchor the γTuRC to the centrosome.
Contenido relacionado
Telophase
Immunization
Hermaphroditism