Triple point
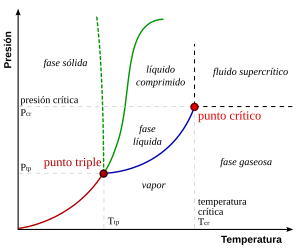
The triple point is the one in which the solid state, the liquid state and the gaseous state of a substance coexist in equilibrium. It is defined with respect to a temperature and a vapor pressure.
The triple point of water, for example, is at 273.16 K (0.01 °C) and a pressure of 611.73 Pa ITS90. This temperature, because it is a constant value, is used to calibrate the Kelvin and Celsius scales of higher precision thermometers.
Triple point of water
It is the combination of pressure and temperature in which the states of aggregation of water, solid, liquid and gas (ice, liquid water and steam, respectively) can coexist in a stable equilibrium. It occurs exactly at a temperature of 273.16 K (0.0098 °C) and at a partial pressure of water vapor of 611.73 pascals (6.1173 millibars; 0.0060373057 atm). Under those conditions, it is possible to change the state of the entire mass of water to ice, liquid water, or steam, arbitrarily, by making small changes in pressure and temperature. Note that even if the total pressure of a system is well above 611.73 pascals (i.e., a system with normal atmospheric pressure), if the partial pressure of water vapor is 611.73 pascals, then the system can still at the triple point of water. Strictly speaking, the surfaces that separate the different phases must also be perfectly flat, to avoid the effects of surface tensions.
Water has an unusual and complex phase diagram (although this does not affect the general considerations about the triple point). At high temperatures, by increasing the pressure, first liquid water is obtained, and then solid water. Above approximately 109 Pa, a crystalline form of ice is obtained. At lower temperatures by virtue of compression, the liquid state ceases to appear and water passes directly from a solid to a gas.
At constant pressures above the triple point, heating ice causes it to change from a solid to a liquid to a gas (or vapor). At pressures below the triple point, such as those found in outer space, where the pressure is close to zero, liquid water cannot exist, and when heated, ice turns directly into water vapor without going through the liquid state., a process known as sublimation.
The pressure of the triple point of water was used during the Mariner 9 mission to Mars as a reference point to define "sea level". More recent missions make use of laser altimetry and gravimetry instead of atmospheric pressure to measure elevation on Mars.
The degrees of freedom that allow us to appreciate more easily the variables of pressure and temperature must be taken into account, at what moment they can be modified and is given by the following equation:
L=C-F+2
Where
L=Degrees of freedom (variables that you can modify).
C=Number of system components.
F= Number of phases of the system.
Pertinent table to know the degrees of freedom on a phase diagram.
| Location | Fases | Degrees of freedom | Meaning |
|---|---|---|---|
| Area | 1 | 2 | 2 variables can be moved |
| About line | 2 | 1 | You can move a variable |
| Triple point | 3 | 0 | No variable can be moved |
Triple points table
This table lists the triple points of some common substances. These data are based on those provided by the National Bureau of Standards (now NIST) of the USA.
| Substance | T(K) | P (kPa) |
|---|---|---|
| Acetilene | 192,4 | 120 |
| Amonia | 195.40 | 6.076 |
| Argon | 83.81 | 68.9 |
| Graphite | 3900 | 10100 |
| Carbon dioxide | 216,55 | 517 |
| Carbon monoxide | 68.10 | 15,37 |
| Deuterior | 18,63 | 17,1 |
| Etano | 89.89 | 8 × 10−4 |
| Ethylene | 104.0 | 0.12 |
| Helio-4 | 2.19 | 5.1 |
| Hydrogen | 13,84 | 7.04 |
| Hydrogen chloride | 158.96 | 13.9 |
| Mercury | 234,2 | 1.65 × 10−7 |
| Methane | 90.68 | 11.7 |
| Neon | 24,57 | 43.2 |
| Nitric oxide | 109.50 | 21.92 |
| Nitrogen | 63.18 | 12.6 |
| Nitrous oxide | 182.34 | 87.85 |
| Oxygen | 54,36 | 0.152 |
| Paladio | 1825 | 3.5 × 10−3 |
| Platinum | 2045 | 2.0 × 10−4 |
| Sulphur dioxide | 197,69 | 1.67 |
| Titanium | 1941 | 5.3 × 10−3 |
| uranium hexafluoride | 337.17 | 151.7 |
| Water | 273,16 | 0.61 |
| Xenón | 161.3 | 81.5 |
| Zinc | 692,65 | 0.065 |
Triple point region
The triple point, as described, is a point on the p - T diagram, so that pressure and temperature of a system located at the triple point are uniquely determined. However, when the system is at this point, it can assume different equilibrium states, as long as neither phase completely disappears. The system can, for example, exchange heat with the surroundings and its volume can change; in the process, the relative proportions of the three phases change. The extensive coordinates of internal energy and total volume are useful as state coordinates. In the U-V diagram, states of simultaneous coexistence of the three phases fill a two-dimensional region, while pressure and temperature are here degenerate to a constant value. Apart from its recognizability, it is precisely this insensitivity to small volume variations and heat input and output, which distinguishes the triple point for its use as a temperature reference.
In a representation of equilibrium states as a surface in a space of pressure - volume - temperature, see diagram, the triple area is reduced to a one-dimensional line, the triple line, which is characterized by the constant values of the triple point for pressure and temperature. Along the triple line, the different volume values correspond to changes in the volume fractions of the phases.
The variability of extensive quantities volume and internal energy does not contradict the Gibbs phase rule, since this rule only makes statements about intensive variables.
Triple Point Cells
Triple point cells are used in the calibration of thermometers. For exact work, triple point cells are typically filled with a high purity chemical such as hydrogen, argon, mercury, or water (depending on the desired temperature). The purity of these substances can be such that only one part in a million is contaminant, called "six 9s" because it has a purity of 99.9999%. A specific isotopic composition (for water, VSMOW) is used because variations in isotopic composition cause small changes in the triple point. Triple point cells are so effective in achieving highly accurate and reproducible temperatures that an international calibration standard for thermometers called ITS-90 relies on hydrogen, neon, oxygen, argon, mercury, and water triple point cells to delineate six of its defined temperature points.
Contenido relacionado
Hardness
Henry cavendish
Lipid

