Toxoplasma gondii
Toxoplasma gondii is a species of eukaryotic obligate intracellular parasitic protozoan. It is the cause of toxoplasmosis, a disease that is generally mild, but that can be complicated and fatal, especially in cats and human fetuses. The cell cycle of T. gondii alternates between sexual reproduction in definitive hosts and asexual reproduction in intermediate hosts. The cat and other felines are considered their definitive hosts because the sexual phase of their life cycle takes place in them. Among the intermediate hosts we can find mammals and birds. It is a very successful parasite and one of the most frequent that infects humans, since it can infect any of the species of homeothermic animals. Humans are common hosts, in 2002 it was estimated that one third of the world population was chronically infected with T. gondii.
Morphology
The different development phases of Toxoplasma gondii show important changes in their morphology, these are called: #Taquizoite, #Bradizoite and #Oocyst.
The Tachyzoite is the form of acute infestation, it is 4-8 micrometers (μm) (micrometers) long and 2-4 μm wide, with a crescent-shaped appearance.
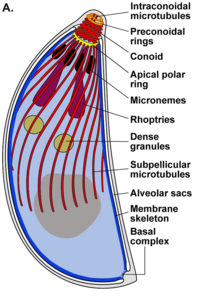
A group of molecular structures, at one end of the T. gondii, known as the apical complex, is essential for both the invasion and the proliferation of this parasite.
Apical complex
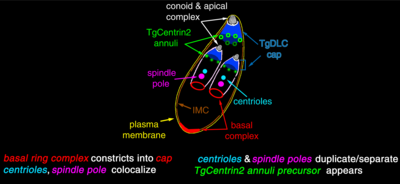
The apical complex (api-complex) of T. gondii, is a complex set of structural and secretory elements at the apical end of the adult parasite, which is built on a set of fibers arranged in a spiral around the conoid. This complex also has three membrane units (as seen in scheme C): an outer plasma membrane (in black) and two membranes in the inner membrane complex (IMC in red) that are the walls of flattened vesicles (alveolar sacs).).
The apical complex provides a semi-rigid framework for the spiky T cells. gondii and is the destination for secretory organelles, which release various invasion factors.
The apical complex is organized around an apical polar ring (APR), which serves as the organizing center and nucleates a series of microtubules, which descend towards the posterior part of the cell.
In addition to its structure, the apical complex acts as a signaling center, being the point of convergence of many regulatory pathways.
Conoid
The conoid of T. gondii, is formed by a compact set of spirally arranged cytoskeletal fibers, which actively move during the invasion stage of host cells.
The structures associated with the conoid are: the pre-conoidal rings, at the distal tip of the conoid, where the conoid fibers originate; the polar ring, from which 22 subpellicular microtubules (MT) originate; and two short intra-conoidal MTs, which can be used as pathways for the transport of secretory vesicles essential for invasion.
After the invasion stage of T. gondii, the so-called parasitophore vacuole (PV) is formed. This is formed both from the plasma membrane of the host cell and from the secretory products of the parasite.
The PV vacuole is isolated from host vesicular traffic and is surrounded by microtubules, endoplasmic reticulum, and host cell mitochondria.
T. gondii also possesses specialized secretory organelles: micronemes (M), rhoptria (R) and dense granules (DG). The sequential secretion of these three types of secretory organelles direct both entry into the host cell and formation of the PV.
Oocyst
An oocyst is the sporulated phase of Toxoplasma. This is a state that can survive outside the host for long periods of time, given its high resistance to the environment. This is the dormant form of the parasite, the product of its sexual reproduction in the intestinal cells of infected cats. These can secrete oocysts up to 1-2 weeks after infection. These oocysts sporulate and become effective in 1-5 days and thanks to their thick cell wall they can survive well in the environment.
Bradyzoite
The bradyzoite (from the Greek brady=slow and zōon=animal) is the slow replication form of the parasite, not only of Toxoplasma gondii, but from other protozoa responsible for parasitic infections. In latent (chronic) toxoplasmosis, the bradyzoite occurs in wall-enclosed microscopic clumps called cysts, in muscle and infected brain tissue.
Tachyzoite
Tachyzoites are motile forms that form pseudocysts in tissues infested with toxoplasma. Tachyzoites are found in vacuoles within cells, this cellular form invades and replicates in infected cells. They activate the immune system and become slowly dividing bradyzoites.
Some of the organelles that can be found in the electron microscopy image on the right are:
apicoplast (A); apical polar ring (APR); conoid (C); conoidal ring (CR); Golgi apparatus (G); inner membrane complex (IMC); Micronemes (M); mitochondria (My); microtubules (Mt); core (Nu); roptries (R); roptria duct (RD); vacuole (V).
Genome and Strains
- Most of the strains T. gondii of the world belong to three different clonal lineages (type I, type II and type III) with minimal genetic differences between them. However, these T. gondii show a varied virulence.
- The T. gondii type I is represented by the GT1 and HR strains (induced by fatal toxoplasmosis in a case of encephalitis in 1939). RH strain has been adapted to in vitro cultivation and is commonly used in laboratory work. Type I is also associated with severe congenital toxoplasmosis in European countries. Type II dominates human toxoplasmosis in the United States is represented by the ME49 strain. Type III is significantly associated with animal guests and is represented by the VEG and CEP strains.
Life Cycle
The life cycle of T. gondii has two phases. The sexual phase of the life cycle occurs only in members of the Felidae family (domestic and feral cats), making these animals the primary hosts for the parasite. The asexual phase of the life cycle can occur in any warm-blooded animal, such as other mammals and birds. Therefore, toxoplasmosis constitutes a parasitic zoonosis.
This protozoan can infect in three ways through:
Tachyzoites: found in pseudocysts, these are released from bradyzoites and penetrate any cell, infecting it until it dies.
Bradizoites: these enter the host in the form of a cyst, where they can release tachyzoites or, in the case of hosts of the Felinidae family, they can form gametes to later form an oocyst.
Oocyst: this last form of infection is only produced in the hosts of the family Felinidae, since it is only in these that the sexual cycle of the protozoan can be carried out. These are formed from the female and male gametes that hatched from the bradyzoites. In these there are 2 sacs with 4 tachyzoites each and they are released in the feces.
The form of infection can range from consuming water or food that is infected with the protozoan, to eating meat with cysts or pseudocysts.
In the intermediate host, including cats, the parasites invade cells, forming a compartment called a parasitophore vacuole that contains bradyzoites, the slowly replicating form of the parasite. The vacuoles form cysts, especially in muscle and brain. Because the parasite is inside cells, the host's immune system does not detect these cysts. Antibiotic resistance varies, but the cysts are difficult to eradicate entirely.
T. gondii propagate within these vacuoles by a series of binary divisions until the infested cell eventually ruptures, releasing the tachyzoites. These are motile, and the asexual reproductive form of the parasite. Unlike bradyzoites, free tachyzoites are effectively eliminated by host immunity, although some manage to infect other cells by forming, thus maintaining the life cycle of this parasite.
Tissue cysts are ingested by the cat when feeding on an infected mouse. The cysts survive passage through the cat's stomach and the parasites infect the epithelial cells of the small intestine where they undergo sexual reproduction and the formation of oocysts, which are shed in the feces. Other animals, including humans, ingest the oocysts (by eating improperly washed vegetables) or tissue cysts by eating raw or improperly cooked meat. T. gondii can infect any type of host cell, with the exception of erythrocytes; This is done by enzymatic action or by allowing itself to be engulfed.[citation required]
Signage between stadiums
The development of encephalic toxoplasmosis occurs due to the transition from a bradyzoite (latent stage) to a tachyzoite (rapidly replicating stage) within the definitive host.
The replication rate of tachyzoites is higher than the rate of bradyzoites and this is because they have a number of factors that speed up their metabolism. Among these factors is the family of Heat Shock Proteins. Proteins, HSP), which contains highly conserved 70kDa low molecular weight proteins (HSP70) and which are a class of ubiquitous chaperones with high immunogenic potential. Until recently, the main function of this class of misfolded proteins was believed to be was to act as intracellular chaperones related to cytoprotection after cells were stressed by external stimuli. However, it is now known that HSP70 not only function as chaperones, but also as cytokines (chaperokines) in the extracellular space.
HSPs are known to be important for bradyzoite differentiation since studies in which the hsp27, hsp70 and hsp90 genes are knocked out suppress the induction of bradyzoites in vitro. On the other hand, when using expression enhancers such as indomethacin, there is an increase in bradyzoite formation. Additionally, in bone marrow cultures, gamma interferons (IFN-ϒ) increased bradyzoite formation due to nitric oxide production. (NO). In addition, T. gondii HSPs (TgHSP70) have been found to be recognized by host B cells (mHSP70, mouse) despite being homologous to each other. Bradyzoite induction then occurs both due to oxidative stress and as a consequence of the host's immune response.
Other discoveries that support the idea that HSP70 are associated with the differentiation of bradyzoites into tachyzoites is that in other protozoa such as Leishmania chagasi and Trypanosoma cruzi, the hsp70 gene has been associated with the ability to survive oxidative stress and lead to differentiation from promastigotes to amastigotes.
It is believed that this differentiation is a response to stress caused by environmental conditions related to the inflammatory response of the definitive host and that the response to heat shock is due to the metabolic plasticity of the parasite during differentiation.
History
In 1908, Charles Nicolle and Louis Manceaux in Tunisia demonstrated the presence of the parasite in a rodent Ctenodactylus gundi, at the same time in Brazil Splendore found it in rabbits. It was named Toxoplasma gondii for toxo (Greek toxon 'arch') for its arched shape; and gondii by mouse.
Transmission of Toxoplasma gondii
Humans can acquire Toxoplasma gondii infection through different routes. First, horizontally through oral ingestion of oocysts from the environment. In addition, horizontally by the ingestion of bradiozites present in raw meats of intermediate hosts. Finally, tachyozites can be transmitted vertically during breastfeeding or transmitted from an infected mother to the fetus during pregnancy. The latter frequently occurs in mothers who have been infected for the first time during pregnancy, this can cause problems in the development of the fetus and pathologies such as psychomotor retardation, hematological abnormalities, hearing and visual loss and can even cause the death of the fetus.
Toxoplasmosis
In immunocompetent individuals, the infection presents with mild symptoms resembling those of the common flu, or they are completely asymptomatic. In this type of patients, the most frequent severe symptoms are retinal infections and lymphadenopathy, which are normally associated with a congenital infection that manifests late.
In immunosuppressed humans, it can act as an opportunistic pathogen, for example in AIDS patients.
Treatment
The use of pyrimethamine and sulfonamides is recommended, the first acting on the synthesis of folic acid and the second on the synthesis of para-aminobenzoic acid (on tachyzoites, not on cysts).
For the prevention of congenital toxoplasmosis in pregnant women, spiramycin is recommended, since it is less toxic. This drug prevents tachyzoites from passing the placental lake to the fetus. The problem with toxoplasmosis in pregnancy is the diagnostic difficulties because the diagnostic techniques for primary infection in pregnant women are not totally absolute or unequivocal. Serological studies are not completely accurate and techniques considered to be of choice in the diagnosis of acute toxoplasmosis such as low avidity IgG obtain non-definitive results in 25% of cases (serological screening in pregnant women). In addition, when it has already been done Once the diagnosis has been made, the transmission has already taken place, and the treatment arrives late to avoid this transmission. The diagnosis is so indeterminate and the secondary effects of medical actions in an indeterminate diagnosis are greater, as well as ineffectiveness with the pharmacological treatment to avoid the transmission to the fetus that it is advocated not to carry out serological screening for toxoplasma in all pregnant women in a systematic way. If the parasite has already crossed the placenta, it is no longer effective, although it seems to have benefits by reducing the parasite load and therefore reducing the severity of symptoms in some cases. In immunocompromised patients, the combination of pyrimethamine with sulfadiazine is recommended, but given the greater possibility of allergy to sulfa drugs in HIV-infected patients, it is sometimes necessary to use the combination of pyrimethamine with clindamycin.
Clinical picture
The acute stage of toxoplasmosis infestations can be asymptomatic, but flu-like symptoms often appear, leading to latent states. Latent infection is also generally asymptomatic, but in immunosuppressed people (transplant patients or with certain infections), they may show symptoms, notably encephalitis, which can be fatal.
Varies depending on which trimester of pregnancy the parasite is acquired.
- 1.♪ trimester: most likely intrauterine fetal death.
- 2.o trimester: the baby is born with malformations.
- 3.♪ trimester: sequelae, severe conditions of the central nervous system, hydrocephalus, reproduces on the walls of the ventricles, there is danger that the necrose tissue obstructs the aqueduct of Silvio, cerebral calcifications, aspect of premature child, hepatoesplenomegaly, jaundice, pneumonitis, myocarditis.
Toxoplasmosis in pregnant women is rarely symptomatic but can cause: lymphadenopathy, fever, myalgia, general malaise, among others.
In humans, chronic latent infection with T. gondii has previously been linked to suicidal self-directed violence, trait aggression and impulsivity, and bipolar disorder.
Regarding the effects in humans, toxoplasmosis can cause schizophrenia. The "biological meaning" of this infection would make sense when humans were hunted by felines, in this case, behavioral alteration promoting aggressive behavior leading to to the social exclusion of the individual, it would increase the chances of being hunted by a feline. In this way, the parasite could carry out its reproductive life cycle in its definitive host.
Infection is also related to a higher suicide rate in infected patients. This could be related to altered dopamine concentration in specific parts of the brain that play an essential role in human behavior and emotions.
Animal behavior
Parasite-host infections are often considered to be an evolutionary mechanism. Both parasite and host develop adaptations and counter-adaptations against each other, resulting in antagonistic coevolution. It has been observed that infection by T. gondii have the ability to change the behavior of rats and mice, making them come closer, instead of running away from the scent of cats. This effect is beneficial to the parasite, which can reproduce sexually if ingested by the cat. A classic example of behavior manipulation is nematomorph-infected crickets. They are manipulated to commit suicide by jumping into bodies of water when infected; in this way, the adult nematomorph is able to complete the reproductive phase of its life cycle, which is aquatic.
The infection is highly precise, in the sense that it does not impact the rat's other fears, such as fear of open spaces or the smell of unfamiliar food. It has been speculated that human behavior may also be affected in some way, and correlations have been found between latent Toxoplasma infections and various characteristics, such as an increase in high-risk behaviours, such as a slowness to react, feelings of insecurity and neurosis that seem to increase suicidality.
Contenido relacionado
Hemicellulose
Leptin
Progesterone