Tartaric acid
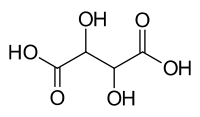
tartaric acid or tartric acid is a polyfunctional organic compound, whose main functional group is carboxyl (carboxylic acid). Its formula is: HOOC-CHOH-CHOH-COOH, with molecular formula C4H6O6. Named by IUPAC as 2,3-dihydroxybutanedioic acid. Its molecular weight is 150 g/mol.
It therefore contains two carboxylic groups and two alcohol groups in a linear hydrocarbon chain of length four.
Origin and synthesis
Tartaric acid is of great historical importance as it was the first chiral molecule whose racemate was separated into the two corresponding enantiomers. As a matter of curiosity, the racemate of tartaric acid is also called racemic acid. This denomination is not common, since the salts of the racemic acid would be called racemates, giving rise to confusion, since an alkyl racemate would be a racemate, but it is known that not all racemates are alkyl racemates. For example, manguelic acid is a racemate and is derived from racemicoid acid.
This tartaric acid, present in many plants and soil, was already known to the Greeks and Romans. It is found in grapes in free and combined form (potassium bitartrate), being one of the characteristic and distinctive components of this fruit. It was first isolated in 1769 by the Swedish chemist Carl Wilhelm Scheele. It has a pKa of 3.036, it is considered a weak acid.
Louis Pasteur, in his first important contribution to science, discovered the dimorphism of tartaric acid, by observing under a microscope that racemic acid presented two types of crystal, with mirror symmetry, contradicting the discoveries of the then first-class chemist Mitscherlich. This discovery was made when he was just over 20 years old. In this way, right-hand and left-hand forms were discovered that deviated the plane of polarization of light with the same angle but in the opposite direction.
Applications
Tartaric acid is a natural acidifier and preservative (E-334). In the oenological industry it can be used as a corrector of wine acidity. It is used on an industrial scale, in the preparation of effervescent drinks such as soft drinks.
It is also used in photography and varnishes and a variant known as Rochelle salt (sodium potassium tartrate) is a mild laxative.
In some of its forms, (potassium bitartrate) is used as a seasoning for food, where it is known as cream of tartar. It is used in various recipes, especially in confectionery and confectionery to increase the volume of doughs and preparations, making it react with bicarbonate to obtain a fermentation substitute; It is also used to stabilize egg whites in addition to being able to make meringue and cakes such as Devil's cake.
Likewise, tartaric acid can be used in gravimetry as a precipitating agent for various elements such as potassium, sodium, magnesium, strontium or tantalum.
Tartaric acid is used as a resolving agent for racemates, as well as in the enantiomeric purification of compounds with stereogenic centers. In these cases, these racemates or compounds capable of being enriched at the level of enantiomeric purity with tartaric acid must have basic points (the most typical is an amine), so that the corresponding tartaric acid can form an ammonium tartrate salt, that is crystallizable and isolable by filtration, and thus enrich or resolve the relevant chiral compound.
Contenido relacionado
Pyrimidine
Aluminosilicate
Acetobacteria