Statin
In pharmacology, statins are known as a group of drugs used to lower cholesterol and triglycerides in their different forms, in patients who have them elevated (for example, with hypercholesterolemia) and who They therefore have a higher risk of developing atherosclerosis and suffering episodes of cardiovascular pathology. From a pharmacological point of view, they are called HMG-CoA reductase inhibitors, although generically and colloquially they are better known by their first name. It is precisely this enzymatic inhibition that produces a decrease in lipoproteins in the body and explains its importance: its positive intervention on cardiovascular risk factors, which lead to numerous cardiovascular pathologies, and which are the main cause of death in the developed world..
Despite their short history (less than forty years), many thousands of studies have been conducted on statins and hundreds of thousands of patients have taken these drugs. This has given rise to a wide knowledge of the characteristics of these drugs that has led to the synthesis of new substances that improve the properties of the previous ones, a line in which a part of pharmaceutical research is still moving. For this, it is enough to know the existence of substances such as Crilvastatin or BAY X 2678, still in the preclinical research phase. However, it has also given rise to broad knowledge of the true toxicological profile of each substance. Phase IV studies have revealed the risks of using these substances for long periods or under certain basal conditions, which has led, among other things, to the withdrawal of a family member from the market due to their higher incidence of side effects. serious secondaries.
As a consequence of the variability in their origin, the pharmacokinetic characteristics of statins present great differences. However, their pharmacodynamic similarities allow them to be grouped for joint study in a natural and useful way. Indeed, regarding the mechanism of action and effects of statins, and, above all, regarding the clinical consequences of their use, there is an important group congruence, which has been widely studied.
History
The discovery of statins is one of the manifestations that best defines modern pharmacology. Initially, the exact therapeutic target where action was desired was defined: HMG-CoA reductase, an enzyme involved in the synthesis of cholesterol. Once it was known, the search for substances that met the requirements for receptor blockade began until the first molecules were found, which were refined until the current statins were achieved.
In 1971, citrinin, a mycotoxin, was found to be a potent inhibitor of HMG-CoA reductase. Subsequently, between 1972 and 1973, it was possible to isolate mevastatin or compactin (substance ML-236B) from fungal cultures, defined as the first statin, but its efficacy to inhibit HMG-CoA reductase was limited. for its toxicity. Later, between 1978 and 1979, the company Merck Research Laboratories developed lovastatin (mevinolin or monacolin K), which was authorized by the FDA for sale to the public in 1987. Mevastatin and lovastatin were obtained from the fungi Penicillium citrinum and Aspergillus terreus, respectively.
Then came pravastatin, a fungal metabolite isolated from cultures of Nocardia autotrophica, and fluvastatin, which was the first fully synthetic statin. Later, simvastatin was synthesized, from a fermentation product of Aspergillus terreus and all the others, maintaining the research in this line at the present time.
Description
Group components
Numerous molecules with HMG-CoA reductase inhibitory properties have been studied. Until 2008, the statins that have passed through the different research filters are, in order of appearance:
- Lovastatin.
- Simvastatin.
- Pravastatin.
- Fluvastatin.
- Atorvastatin.
- Cerivastatin, market withdrawal in 2001 by the manufacturer laboratory (Bayer) due to its association with serious adverse effects
- Rosuvastatin.
- Pitavastatin.
All of them are recognized by the FDA in the United States and the AEM in the European Union.
Pharmacokinetics
Route of administration
So far all statins developed are used orally.
Absorption
They are absorbed orally, in a variable range from 30% for lovastatin to 35% for pravastatin. As a general rule, statins decrease their absorption in the presence of food in the stomach. However, the modifications in the maximum concentrations or in the respective assimilation curves, have no repercussion on the final results in the modification of cholesterol levels, so in general it is advisable to take them at any time of the day and in most of the cases with or without food. Likewise, there does not seem to be accumulation due to multiple doses, so it is a general consensus to take it in a single dose. The recommendation not to drink grapefruit juice while taking statins is due to interference with metabolism, not absorption disturbances.
In general, the bioavailability of statins is poor, ranging from 5% for lovastatin to 17% for pravastatin.
Distribution
Binding to plasma proteins is variable, but generally very high. Except for 50% of pravastatin all are above 95%. The tissue distribution is extensive, crossing the blood-brain and placental barriers, even passing into milk in lactating women.
The hepatic specificity of these drugs is determined by their degree of lipophilicity and by the presence of organic anion transporting proteins that allow more hydrophilic statins, such as pravastatin and rosuvastatin, to enter the hepatocyte. some statins can inhibit multidrug resistance protein (p-glycoprotein), a protein that transports many drugs in the cell, and therefore could predispose to drug interactions.
Metabolism and metabolites
The metabolism is hepatic, undergoing first pass effect. In the majority, there are differences in metabolization with respect to sex and age, but not enough to modify the doses in the absence of other pathologies. It seems clear that they are CYP450 substrates: lovastatin, simvastatin, and atorvastatin are metabolized exclusively by CYP3A4, and fluvastatin is exclusively metabolized by 2C9. In the case of rosuvastatin, only 10% use CYP2C9 and 2C19. Pitavastatin has a low affinity for CYP2C9, so it does not represent an important pathway of metabolism. Pravastatin is not metabolized by the cytochrome pathway, but rather by enzymes present in the cytoplasm of the hepatocyte.
The metabolites can be hydroxylated, omega or beta-oxidized, methylated or glucuronidated derivatives. Their pharmacological activity is highly variable. Thus, the range is wide, from lovastatin or simvastatin, which are really pharmacologically inactive lactones and carry out their pharmacological activity through their metabolites, to fluvastatin, which has practically inactive metabolites.
Excretion
For the most part, excretion is in the feces, due to poor absorption. Depending on each type of statin, renal excretion ranges from 2% to 20%.
As a summary we can see in the following table the differences and similarities between the statins:
| Substance | Simvastatin | Pravastatina | Lovastatin | Fluvastatin | Atorvastatin | Rosuvastatin | Pitavastatin |
| Professors | Yes | NO | Yes | NO | NO | NO | NO |
| Food and absorption | They don't influence. | Slow down. | Increase | Slow down. | Slow down. | They don't influence. | - |
| Bioavailability | ≤ 5 | 18% | ≤ 5% | 24 per cent | 14% | 20% | ▪0% |
| Union to plasma proteins | 94% | 50% | | 98% | 98% | 88% | - |
| Across a hematoencephalic barrier | Yes | NO | Yes | NO | NO | NO | Yes |
| Metabolism | CYP3A4 | Sulfat | CYP3A4 | CYP2C9 | CYP3A4 | CYP2C9 | CYP2C9; CYP2C8 |
| Excretion biliar | 60% | 70% | 83% | 95% | - | 90 per cent | - |
| Urinary exemption | 13% | 20% | 10% | 5% | 2% | 30% | 3% |
| Semivida | 2-3 h. | 0.8 h. | 1-4 h. | 2.5 h. | 20 h. | 20 h. | - |
Pharmacodynamics
Mechanism of action
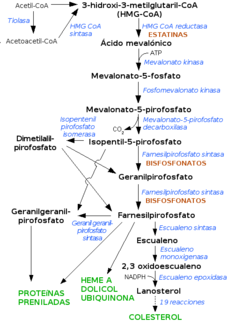
Statins are 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) inhibitors. This enzyme catalyzes an essential step in the mevalonate pathway, the conversion of HMG-CoA to mevalonate, which is a key metabolite in cholesterol biosynthesis. Its blocking occurs due to the great structural similarity that these drugs exhibit with HMG-CoA. The affinity of statins for the enzyme is 1,000 to 10,000 times that of the natural substrate. The attached diagram shows the blocking level of statins as well as other substances in the biosynthesis of cholesterol.
The concrete reaction would be:
![]()
In which an HMG-CoA molecule is reduced by the action of HMG-CoA reductase and the coenzyme NADPH, resulting in mevalonate and CoA. The inhibition of statins is carried out in a competitive, partial and reversible way.
Blocking the hepatic synthesis of cholesterol causes activation of the regulatory proteins SREBP (sterol regulatory elements-binding proteins), which activate the transcription of proteins and, therefore, they produce increased expression of the LDL receptor gene and an increase in the number of functional receptors in the hepatocyte.
On the other hand, statins have also been shown to inhibit lymphocyte function-associated antigen-1 (LFA-1). LFA-1 is a glycoprotein of the family of integrins expressed by the surface of leukocytes. When LFA-1 is activated by certain receptors, it binds to intracellular adhesion molecule-1 (ICAM-1 or CD 54) and stimulates leukocyte extravasation and T-lymphocyte activation This means that LFA-1 is a proinflammatory agent and that its inhibition is beneficial in processes such as rheumatoid arthritis and homograft rejection. Statins, and especially lovastatin, have been shown to bind to a site in the LFA-1 domain, now designated the lovastatin site. This is the molecular mechanism by which lovastatin, simvastatin and, to a lesser extent, other statins, inhibit LFA-1. It would be one of the anti-inflammatory and, therefore, antiatherogenic mechanisms that statins have.
Effects
In summary, the consequences of HMG-CoA inhibition can be grouped into two large groups:
A) Derived from the interaction on cholesterol metabolism
- Total cholesterol and LDL levels decrease, substances closely related to atherosclerosis and increased cardiovascular risk.
- It decreases the density of LDL particles, increasing the size of these, leading to a decrease in atherogenesis.
- Apolipoprotein B also decreases substantially during treatment with statins.
- In addition, some statins moderately increase cHDL and reduce plasma triglycerides. As a result of these changes, the quotient between total cholesterol and HDL cholesterol, as well as the quotient between LDL cholesterol and HDL cholesterol are reduced. It has been considered the combination with fiber as a booster of the heart prevention of statins, especially for not having competitive pathways of metabolism.
B) Pleiotropic effects: Apart from their effects on the lipid profile, statins have other beneficial cardiovascular effects, especially on the arterial wall, known as pleiotropic effects and which would explain the non-attributable additional benefit to the reduction in LDL-C observed in many intervention studies.
- By inhibiting HMG-CoA reductase, statins interfere with isoprenoid formation from mevalonate. Isoprenoids are molecules, such as pharynylpirophosphate (FPP) and geranylgeranylpirophosphate (GGGPP), derived from mevalonate metabolism, which serve as lipidic labels for the post-translation of a wide variety of proteins, including the gamma subunit of G proteins and GTP small binding proteins. As a result, the priority of G proteins (Rho, Rac, Rac1 Rab and Ras) is reduced. The priority of these molecules is necessary for their anchor to the cell membrane and, thus, be able to exercise their mechanism of action related to migration, differentiation and cell proliferation. In general, they stimulate pro-inflammatory pathways and inhibit useful mechanisms for endothelium homeostasis.
- Through these potential effects on cellular proteins, statins may have a number of antiaterosclerotic and antitrombotic properties, such as inhibiting the growth of the smooth muscle cell, cell adhesion, platelet activation, and reactive C protein secretion, among others.
- Mevalonic acid can also act directly by inhibiting the synthesis of nitric oxide (NO), in a process dependent on the inhibition of the transferase genilgeranil. NO is an essential molecule for the proper function and vasodilation of the endothelium.
- To this we must add the effects resulting from the inhibition of LFA-1, which ends up in an important way affecting the endothelial function of the blood vessels.
These pleiotropic effects are a constant source of research, since they can broaden the profile of use of statins. For this reason, they are developed in greater depth below.
Statins and endothelial function
Statins maintain and improve endothelial function by increasing the bioavailability of nitric oxide, which is synthesized by the enzyme NO synthetase (eNOS). Nitric oxide is the main regulator of arterial homeostasis and endothelium-dependent vasodilatation. The functions of NO are, among others, the inhibition of pro-inflammatory mechanisms and acting as an antioxidant on lipoproteins. Statins preserve and increase the bioavailability of NO in several ways:
- Rho protein inhibition increases the expression of the sintetase enzyme of nitric oxide.
- Increasing the half-life of the RNA messenger of the sintetase enzyme of nitric oxide.
- Reducing excess cavernolin, a molecule that acts as an inhibitor of the sintetase enzyme of nitric oxide.
- Inhibiting the production of superoxide.
By protecting NO, statins decrease platelet aggregation and reduction of thromboxane A2 by platelets and thus limit the formation of unstable plaque. Statins also increase the expression of tissue plasminogen activator and inhibit the expression of endothelin-1, a potent vasoconstrictor with mitogenic action.,
Antioxidant properties of statins
The lipid-lowering action itself reduces oxidative stress. However, statins have their own antioxidant mechanisms that inhibit the production of the superoxide anion radical. Superoxide is synthesized by NADPH oxidase, an enzyme that can be activated by the action of the membrane receptor for angiotensin II, type I (R-AT1). Statins block R-AT1 and also inhibit NADPH oxidase phosphorylation, inactivating it.,
Inhibition of smooth muscle proliferation
Smooth muscle proliferation is a central phenomenon in the pathogenesis of vascular lesions, including postangioplasty restenosis, posttransplant atherosclerosis, and occlusion of coronary vein grafts. Statins block RhoA, one of the mediators of smooth muscle proliferation.
Anti-inflammatory action
Atherosclerosis has a strong inflammatory component characterized by the presence of monocytes, macrophages, and T lymphocytes in the plaque. This process is induced by proinflammatory cytokines, free radicals, and nitric oxide deficiency. Statins, in addition to increasing the bioavailability of nitric oxide, inhibit several of the proinflammatory cytokines. A marker of inflammation and also a prognostic factor for coronary heart disease risk is C-reactive protein (CRP). CRP is also considered to be pro-inflammatory, since by binding to the LDL-C of the atheromatous plaque, it activates complement and induces the expression of plasminogen activator inhibitor 1 (PAI-1).), reduces the expression of eNOS and increases the expression of adhesion molecules., Therefore, it is valid to assume that the decrease in plasma CRP values could be beneficial. Large studies with statins, such as AFCAPS/TexCAPS, showed a reduction in CRP in blood. Due to their anti-inflammatory action, statins increase the stability of the atheromatous plaque, and much of the reduction in coronary complications is attributable to this mechanism. Preclinical studies demonstrated that statins reduce the accumulation of macrophages in the atheromatous plaque and inhibit the production of metalloproteinases by activated macrophages. Metalloproteinases have the ability to degrade supporting proteins and are therefore partly responsible for the accident of plaque with thrombus formation.
Clinically, the effects of statins lead to a decrease in cardiovascular risk, thus being able to say that there are five mechanisms by which statins could prevent cardiovascular diseases:
- Decreasing cholesterol levels directly.
- Improved endothelial function
- Modulating the inflammatory response
- Stabilizing the ateroma plate
- Preventing the formation of the thrombo
As a summary, the following diagram shows the different mechanisms of action of statins and relates them to clinical effects.
Clinical trials with statins
Since the publication of studies such as the Framingham Heart, the Seven Countries or the MRFIT (Multiple Risk Factor Intervention Trial), the role of hypercholesterolemia as the main risk factor in cardiovascular morbidity and mortality episodes became clear.,, With studies such as the Lipid Research Clinics Coronary Primary Prevention or the Helsinki Heart showed that cholesterol reduction prevented the occurrence of these cardiovascular events. This led to the creation of the NCEP (National Cholesterol Education Program) in 1987 and its recommendations on the treatment of hypercholesterolemia. Angiographic studies were initiated to anatomically corroborate the findings of these studies, and studies with statins began following the NCEP recommendations. Since then, numerous studies have been carried out, highlighting the following:
- Studies in Primary Prevention:
- Study WOSCOPS (WOSCOPS)West Of Scotland Coronary Prevention Study), in 6695 male patients and performed with pravastatin and with recent publication of long-term results.
- AFCAPS StudyAir Force Coronary Atherosclerosis Prevention Study), with 6605 patients and performed with lovastatin.
- CARDS studyCollaborative Atorvastatin Diabetes Study), with 2838 patients treated with atorvastatin.
- Estudio CHESS (Study CHESS)Comparative HDL Efficacy and Safety Study), performed on 917 patients, comparing simvastatin to atorvastatin at high doses.
- PRINCE StudyThe PRavastatin INflamation/CRP Evaluation), with 1702 patients and assessment of the anti-inflammatory effects of pravastatin. It includes a substudy on secondary prevention.
- JUPITER Study (JUPITER)Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin), ongoing study that aims to study in 15000 patients the effectiveness of rosuvastatin versus placebo.
- STELLAR StudyStatin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin). Open randomized study that compares the efficacy of rosuvastatin versus atorvastatin, pravastatin and simvastatin at variable doses in patients with primary hypercholesterolemia.
- ADVOCATE Studythe ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation)
- Study ORBITALOpen label care primary study: Rosuvastatin Based compliance Initiatives linked to achievement of LDL goals). Randomized, open, 24-week study that values the efficacy of rosuvastatin alone or in combination in patients with primary hypercholesterolemia, according to European LDL cholesterol targets.
- FDF study (the French-Dutch Fluvastatin study). Randomized, double-blind and control group study, with 431 patients with primary hypercholesterolemia and assessment of the effects of fluvastatin on LDL and HDL levels.
- Studies in Secondary Prevention:
- Study 4S (Scandinavian Simvastatin Survival Study), with 4,444 patients with coronary heart disease and hypercholesterolemia.,
- CARE studyCholesterol and Recurrents Events Study), with 4159 patients and pravastatin.
- LIPID Study (LIPID)Long-term Intervention with Pravastatin in Ischemic Disease), with 9014 patients and also pravastatin.
- Cardiac Protection Study or HPSHeart Protection Study), with a total of 20,536 patients and study of simvastatin at doses of 40 mg/day in 5963 of them, in which it reduced the risk of coronary disease and total cardiovascular events both in diabetics with a history of coronary disease and in which they did not have the antecedent.
- MIRACL Study (MIRACL Study)Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study), with 3086 patients and high doses of atorvastatin.
- ASCOT-LLA Study (ASCOT-LLA)Anglo-Scandinavian Cardiac Outcomes Trial- Lipid Lowering Arm), with 10305 patients and study of atorvastatin.
- PROVE IT Study (PROVE IT)Pravastatin or Atorvastatin Evaluation and Infection therapy-Thrombolysis in myocardial Infarction), with 4162 patients and comparison of atorvastatin at high doses with pravastatin.
- TNT study, (Treating to New Targets), with 10001 patients and comparison between high and low doses of atorvastatin.
- Study 3T (Treat-To-Target Study), with 1087 patients with cardiovascular disease and dyslipemia, treated with atorvastatin or simvastatin.
- Study ALLHAT-LLT (The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) with 10355 hypertensive and dyslipemic patients, using pravastatin (40 mg per day).
- Estudio COMETS (Commerce Study)COmparative study with rosuvastatin in subjects with METabolic Syndrome). Randomized, 12-week double-blind study that compares the effect of rosuvastatin against atorvastatin and placebo on LDL cholesterol levels in patients with metabolic syndrome and cardiovascular risk to 10 years less than 10%.
- ECLIPSE StudyAn Evaluation to Compare Lipid lowering effects of rosuvastatin and atorvastatin In force titrated subjects: a Prospective Study of Efficacy and tolerability). 24-week randomized study that compares the efficacy of rosuvastatin and atorvastatin in patients with primary hypercholesterolemia and cardiovascular risk at 10 years older than 20%.
- EXPLORER Study (Experience Study)EXamination of Potential Lipid-modifying effects Of Rosuvastatin in combination with Ezetimibe versus Rosuvastatin alone). 6-week open randomized trial that compares the efficacy of rosuvastatin in combination with ezetimiba versus rosuvastatin alone in patients with hypercholesterolemia and cardiovascular risk at 10 years older than 20%.
- MERCURY I StudyMeasuring Effective Reductions in Cholesterol Using Rosuvastatin therapY I). 16-week open randomized study that compares the efficacy and safety of rosuvastatin against atorvastatin, pravastatin and simvastatin in patients with primary hypercholesterolemia, following European parameters.
- MERCURY II StudyMeasuring Effective Reductions in Cholesterol Using Rosuvastatin therapY II). 16-week open randomized study that compares the efficacy and safety of rosuvastatin against atorvastatin and simvastatin in patients with primary hypercholesterolemia, following the parameters of NCEP ATP III.
- PULSAR StudyProspective study to evaluate the Utility of Low doses of the Statins Atorvastatin and Rosuvastatin). Open randomized study that compares the safety and efficacy of rosuvastatin and atorvastatin at low doses in patients with hypercholesterolemia and cardiovascular risk at 10 years older than 20%.
- POLARIS StudyProspective Optimisation of Lipids by Atorvastatin or Rosuvastatin Investigated in high-risk Subjects with hypercholesterolaemia). 26-week randomized double-blind study that compares the efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and cardiovascular risk at 10 years older than 20%.
- CORONA Study (CORONA Study)COntrolled ROsuvastatin multiNAtional trial in heart failure). Randomized, double-blind study, which evaluates the effect of rosuvastatin in 5011 patients with ischemic heart failure.
- AURORA StudyA study evaluating the Use of Rosuvastatin in patients requiring Ongoing Renal dialysis: an Assessment of survival and cardiovascular events). Randomized, long-term double-blind study with 2775 patients comparing rosuvastatin to placebo.
- DISCOVERY Study (Discovery Study)DIrect Statin COmparison of LDL-C Values: an Evaluation of Rosuvastatin therapY). Randomized, open, 12-week study, designed to evaluate the effectiveness of rosuvastatin against other statins in patients with primary hypercholesterolemia according to European targets of LDL cholesterol levels.
- LUNAR StudyLimiting UNdertreatment of lipids in ACS with Rosuvastatin). Randomized, open, 12-week study that compares rosuvastatin to atorvastatin in patients with acute coronary syndromes.
- URANUS Studythe Use of Rosuvastatin versus Atorvastatin iN type 2 diabetes mellitUS). Randomized, double-blind study, which compares in type II diabetic patients the LDL cholesterol response to treatment with rosuvastatin or atorvastatin.
- Study ANDROMEDAA raNdomized, Double-blind study to compare Rosuvastatin [10 & 20 mg] and atOrvastatin [10 & 20 Mg] in patiEnts with type II DiAbetes).
- Study CORALL (COmpare Rosuvastatin [10–40 mg] with Atorvastatin [20–80 mg] on apo B/apo A-1 ratio in patients with type 2 diabetes meLLitus and dyslipidaemia).
- Angiographic studies:
- Post-CABG StudyPost- Coronary Artery Bypass Grafting Trial), with 1351 patients and the use of lovastatin.
- AVERT Study (AVERT Study)Atorvastatin versus Revascularization Treatment), with 341 patients and using atorvastatin.
- REGRESS studyThe REgression GRowth Evaluation Statin Study), with 600 patients and using pravastatin.
- ACAPS Study (ACAPS Study)the Asymptomatic Carotid Artery Progression Study), with 919 patients and study of lovastatin.
- CCAIT Study (CCAIT)Canadian Coronary Atherosclerosis Intervention Trial). 331 patients with lovastatin, which led to an interesting substudy in women.
- PLAC I StudyPravastatin, Limitation of Atherosclerosis in the Coronary arteries I), with 408 patients.
- PLAC II StudyPravastatin, Lipids, and Atherosclerosis in the Carotid arteries II), with 151 patients and evaluation of pravastatin.
- EStudio KAPSKupio Atherosclerosis Prevention Study), with 447 patients treated with pravastatin.
- Studio MARS, (the Monitored Atherosclerosis Regression Study), with 188 patients and assessment of lovastatin.
- ASAPS Study (ASAPS Study)the Atorvastatin vs. Simvastatin on Atherosclerosis Progression Study), with 325 patients.
- BCAPS Studyß-Blocker Cholesterol-lowering Asymptomatic Plaque Study), with 793 patients and fluvastatin, although it mainly studies the effectiveness of metoprolol to decrease the atheroma plate of carotid.
- CAIUS Study, (the Carotid Atherosclerosis Italian Ultrasound Study), with 305 patients and investigating pravastatin.
- FAST StudyFukuoka AtherosclerosiS Trial), with 246 patients comparing pravastatin and probucol.
- ARBITER Study(s)Arterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol), with 141 patients comparing atorvastatin and pravastatin.
- Study ARBITER 2 indirect study in which the effectiveness of treatment with niacin associated with statins is valued.
- REVERSAL Studythe REVERSing atherosclerosis with Aggressive Lipid lowering study), comparing pravastatin and atorvastatin in 654 patients.
- IDEAL Studythe Incremental Decrease in Endpoints through Aggressive Lipid lowering trial), comparing atorvastatin and simvastatin in 8888 patients over the age of 80.
- SEARCH Study (SEARCH Study)the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine tests), comparing low and high doses of simvastatin.
- ASTEROID Study (ASTEROID)A Study To Evaluate the effect of Rosuvastatin On Intravascular ultrasound-Derived coronary atheroma burden), Open 24-month study designed to evaluate the effects of rosuvastatin on the thickness of the coronary atheroma plate in 349 patients.
- METEOR StudyMeasuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin). Randomized, double-blind, 24-month-long study that evaluates the effect of rosuvastatin on carotide atheroma plaque in asymptomatic patients with low-risk hypercholesterolemia.
- Estudio ORION (Outcome of Rosuvastatin treatment on carotid artery atheroma:a magnetic resonance Imaging ObservatioN). Randomized, double-blind, 24-month-long study evaluates the effect of rosuvastatin at different doses on atheroma carotydea plaque in asymptomatic hypercholesterolemic patients.
- LIPS StudyLescol Intervention Prevention Studyrandomized, double-blind trial with control group, performed on 1658 patients with chest angina, and evaluating the effectiveness of fluvastatin to reduce the risk of major heart events.
- Study of the 3 provinces. An interesting retrospective study carried out in 3 provinces of Canada with 18,637 patients with previous myocardial infarction, in which the effectiveness of 5 statins (atorvastatin, pravastatin, simvastatin, lovastatin and fluvastatin) was compared in secondary prevention.
- Other angiographic studies with monotherapy statins, such as MAAS, CIS or LCAS.
Innumerable other clinical trials have been carried out to try to demonstrate the usefulness of statins in other pathologies, such as osteoporosis, Parkinson's disease, Alzheimer's disease, or septic shock. , , , At the 2008 American College of Chest Physicians Annual Meeting (CHEST 2008, October 2008, Philadelphia), Danai Khemasuwan and colleagues from the Albert Einstein Medical Center presented the results of a study showing that statin therapy is associated with a reduction in venous thromboembolism, a disorder that includes deep vein thrombosis and pulmonary embolism, in patients with solid tumors in organs such as the breast, lung, and colon. The results revealed that patients taking statins were less likely to develop venous thromboembolism compared to the rest (8% vs. 21%). This effect was independent of other factors, such as smoking, metastasis, chemotherapy used, immobility, and use of aspirin.
Interactions
Statins, like many drugs, can interact with other drugs and substances.
Pharmacodynamic interactions
Both fibrates and niacin (nicotinic acid) increase the risk of statin-associated myopathy. This is especially important in the case of gemfibrozil, which, in addition to interacting at the receptor level, interferes with hepatic metabolism at the CYP3A4 level.
Pharmacokinetic interactions
The most important interactions of statins occur at the level of hepatic metabolism, since several of them depend on different CYP450 isoenzymes. Thus, while lovastatin, simvastatin, and atorvastatin depend on CYP3A4, and rosuvastatin depends on CYP2C9, the enzyme involved has not been defined for fluvastatin and pravastatin, although some of their pharmacokinetic interactions are known. Thus, in relation to hepatic metabolism, we can find the following interactions of interest:
Powerful
| Other
grapefruit juice)
non-nucleoside:
|
|
|
|
| Burmese. | Interaction results. |
|---|---|
| Antiacids. | Reduce the absorption of statins. |
| Anticoagulants. | It increases the anticoagulant effectiveness. |
| Ionic exchange resins. | Reduce the absorption of statins. |
| Colchicina. | Increased toxicity of colchicin. |
| Glibenclamide. | Increased plasma levels of glybenclamide. |
| Oral contraceptives. | Up to 30% increase hormone levels in blood. |
Clinical use
Indications
- Dislipemias.
Statins are indicated as a dietary supplement to reduce elevated levels of total cholesterol, LDL cholesterol, Apolipoprotein B, and triglycerides; and to increase HDL cholesterol in patients with:
- - Primary hypercholesterolemia.
- - Mixed dyslipidemia.
- - Homozygotic family hypercholesterolemia.
- Cardiovascular prevention.
"In people with low cardiovascular risk, a statin does not reduce all-cause mortality or severe cardiovascular morbidity."
- Primary prevention of coronary events. In hypercholesterolemic patients without clinical evidence of coronary disease:
- - Reduce the risk of myocardial infarction.
- - Reduce the risk of myocardial revascularization procedures.
- - Reduce the risk of cardiovascular mortality without an increase in the death of non-vascular causes.
- Secondary prevention of cardiovascular events. In patients with clinical evidence of cardiovascular diseases:
- - Reduce the risk of total mortality by reducing coronary death.
- - Reduce the risk of myocardial infarction.
- - Reduce the risk of myocardial revascularization procedures.
- - Reduce the risk of stroke and transient ischemic attacks (TIA).
- - To slow progression of coronary atherosclerosis.
- Supplementary treatment to the correction of other risk factors and other cardioprotective treatments.
Adverse effects
In general, statins are well tolerated and the dropout rate in clinical trials due to any adverse effect is < 10%, similar to that of patients taking placebo, and less than 1% are serious adverse effects. The most serious adverse effect is related to muscle disease, which can range from myalgia (proximal muscle pain and/or muscle weakness with a normal or slightly increased creatine kinase [CK] value) to more severe forms, such as myopathy (pain and/or weakness plus the presence of very elevated CK, generally > 10 times the normal value) or rhabdomyolysis (severe muscle condition, with muscle weakness and pain, presence of very elevated CK, myoglobinuria and renal failure). In general, the most common condition is myalgia without CK elevation. A separate mention deserves cerivastatin, currently withdrawn from the market, since it is the statin that caused the greatest number of severe cases of myopathy. The rate of fatal rhabdomyolysis associated with the use of cerivastatin was at least 15 times higher than that produced by other statins, and was related to the use of high doses of the drug (0.8 mg/day) or when it was co-administered with gemfibrozil.
As new statin molecules have been synthesized, efforts have been made to improve therapeutic efficacy on the one hand, but also to reduce the occurrence of adverse reactions on the other. Therefore, the frequency of their appearance must be taken as a guide, consulting the frequencies at the individual level in each case. For the assessment of adverse reactions (RAM) we will take into account the criteria of the CIOSM.
| System involved. | CIOSM Group. | Type of reaction. |
| Gastrointestinal disorders. | Frequent. | Constipation, flatulence, dyspepsia, nausea, diarrhea. |
| Very rare. | Anorexia, vomiting. | |
| Rare. | Hepatitis, cholestatic jaundice. | |
| Very rare. | Hepatic insufficiency. | |
| Disorders of the nervous system. | Frequent. | Headache, dizziness, paraesthesia, hypoesthesia. |
| Very rare. | Peripheral neuropathy. | |
| Very rare. | Disgeusia. | |
| Leather and rabbits. | Frequent. | Skin rash, prurito. |
| Very rare. | Urticaria. | |
| Very rare. | Angioneurotic eruption (including multiform erythema, Stevens-Johnson syndrome and toxic epidermal necrolysis). | |
| Musculoskeletal disorders. | Frequent. | Mialgias, artralgias. |
| Very rare. | Miopathy. | |
| Rare. | Miositis, rhabdomiolysis, muscle cramps. | |
| Very rare. | Trendy break. | |
| Endocrine disorders. | Very rare. | Alopecia, hyperglycemia, hypoglycemia, pancreatitis. |
| Psycho. | Frequent. | Insomnia. |
| Very rare. | Amnesia. | |
| General disorders. | Frequent. | Astenia, chest pain, back pain, peripheral edema, fatigue. |
| Very rare. | Weight, weight gain. | |
| Several. | Frequent. | Allergic reactions. |
| Very rare. | Trombocytopenia, hear us, impotence. | |
| Very rare. | Visual or auditory alterations, gynecomastia, anaphylaxia. | |
A study, published in the July 31, 2007, issue of the Journal of the American College of Cardiology, has brought to light another surprising epidemiologic association: low LDL cholesterol levels are associated with increased cancer risk.[citation needed] It is so surprising that it must be contrasted, especially considering large meta-analyses that have been carried out and obtained totally different results.
Contraindications
The following will be absolute contraindications:
- Hypersensitivity to any statin or to some of the excipients of commercial presentations.
- Active hepatopathy or persistent and inexplicable elevations of serum transaminates
- Pregnancy and breastfeeding.
- Concomitant administration of CYP3A4 potent inhibitors (itraconazole, ketoconazole, HIV protease inhibitors, erythromicin, clarythromycin, telithromycin and nephazodone) or CYP2C9. (Contraindication in non-CYP450 statins).
These will be relative contraindications (it can be taken but special medical supervision will be necessary):
- Elders (age oriented 70 years).
- Kidney failure.
- Hypothyroidism without control.
- Personal or family history of inherited muscle disorders.
- History of muscle toxicity with a statin or fiberte.
- Alcoholism.
- Concomitant administration of CYP3A4 weak inhibitors.
Introductions
Most of the presentations are in the form of tablets. In some cases these are coated. Fluvastatin comes in a sustained-release form.
Given the physicochemical characteristics of statins, among the usual excipients we can find:
- Gelatina,
- Magnesium stearate,
- Cellulose microcrystalline (E-460 I)
- Pregelatinized starch (maize),
- Lauril sulfato de sodio,
- Talco,
- Titanium dioxide (E171)
- Red iron oxide (E172)
- Yellow iron oxide (E172)
- Black iron oxide,
- Bench alcohol,
- Butilparaben, methylparaben and propil paraben.
- Sodium carboximetilcellulose, (E468)
- Calcium phosphate edetate, (E540)
- Silicon dioxide,
- Sodium propionate. (E281)
- Potassium bicarbonate (E501)
- Povidona, (E1201)
- Polyethylene glycol 8000.
Contenido relacionado
Bronchiectasis
Amniocentesis
Simvastatin