Spermatophyte
The spermatophytes or phanerogams (Spermatophyta) are a monophyletic group of the kingdom of plants (Plantae) that includes all the lineages of vascular plants that produce seeds.
The scientific name comes from the Greek σπέρμα ("sperma", meaning "seed"), and φυτόν ("phyton", meaning " planta"), which translates as “seed-bearing plants”. The circumscription of the group (ie the taxa of which it is composed) exactly matches that of the ancient taxon Phanerogamae, which is therefore synonymous with this division. Because in spermatophytes the pollen grain produces a tube (haustorial or pollen) to reach the ovule and fertilization to occur, this group is also called siphonogamous embryophytes (from the Greek: embrios: embryo; phyton: plant; xiphos: tube; fallen deer: sexual union. Literally, "plants with embryo whose sexual union occurs with tube"). This group is sometimes referred to in scientific jargon as 'embryophytes', leaving out asiphonogamous embryophytes or bryophytes and ferns and the like.
Scientists have long agreed on the monophyly of spermatophytes. Among the morphological evidence of spermatophyte monophyly is, of course, the seed itself, and also the production of wood (or "secondary xylem" generated in the secondary meristem called "cambium"), at least in ancestral form. Another notable feature is axillary branching, compared to the anisotonic dichotomous branching of its euphyllophyte ancestors.
Spermatophytes originated at the end of the Devonian, from lignophytes, which already had wood production and axillary ramification, as can be seen in the fossil record.
Today spermatophytes are by far the largest lineage of vascular plants, with some 270,000 living species. A single subclade is most responsible for this diversity: the angiosperms, or periantate flowering plants. Other subclades, usually grouped as gymnosperms, are the cycads, the ginkgos, the conifers, and the gnetals. These four groups share a common ancestor. They are also called "gymnosperms" to some fossils of spermatophytes that do not produce perianate flowers, which do not share the same ancestor as living gymnosperms, which is why some authors differentiated "Gymnospermae sensu lato" (pteridosperms + living gymnosperms), which would be paraphyletic with respect to angiosperms and "Gymnospermae sensu stricto", monophyletic, comprised by living lineages.
Synonymy
This group has been named Phanerogama (Bartling 1830), Phanerogamae (Brongniart 1843, Eichler 1883), Spermatophyta (Willkomm 1854, Goebel 1882, Britton & A. Brown 1896), Anthophyta (Braun in Ascherson 1864, Wettstein 1924- 1935), Siphonogamae (Engler 1886), Embryophyta siphonogama, (Engler 1892-1924), Magnoliophyta division (Takhtajan 1964) and Spermatophytina (Cavalier-Smith 1998, Ruggiero et al. 2015).
Life cycle and reproductive structures of spermatophytes
Spermatophytes can be defined as tracheophytes with the following characteristics:
- As in all tracheofites, there are two alternate multicellular generations, called gametophyte and sporophyte, being the taloid gametophyte, and the sporophyte organized in tissues and organs. Spoophyte is a "corm" (with vascular system, root and stem). As in all the euphyllites, the sporophyte stem grows thanks to its apical meristhema, and it is branched as a main stem with lateral branches, and also has leaves ("euphiles").
- In spermatophytes the alternation of gametophytic and sporophytic generations is given in masked form, since the gametophyte develops completely within the structures of the sporophyte (even in the most primitive groups can still be seen beforehands and archgons).
- They possess a cycle of heterospore life, with endosporic development gametophyte (the sporophyte gives female spores within which the female gametophyte is developed, and male spores within which the male gametophyte is developed).
- Sporangios are always born in the leaves (the fertile leaves are called "sporophiles"). In spermatophytes, fertile leaves, sporang carriers, are always on a limited growth branch ("brachiblast"). Therefore in the corm appears a new structure, characteristic of spermatophytes: the flower (a defined growth branch carrying fertile leaves). Morphologically in the flower can be differentiated an axis that originates in the stem and which presents one or two bracts, which rows in a receptacle where the megasporophiles that will give the megasporas, and the microsporophiles or stamens that will give the microspores. The megasporophiles and the microsporophylls, together are the cravings, so this taxon is also known as Antophyta (but it is not advisable to use that name, because some authors consider it as "sophites" only to angiospermas and gnetals, which were previously believed to have a common predecessor). In the flower we will see the "masked" development of the gametophyte:
- in the flower the fertile leaves of megasporangios (the "megaesporophiles") will contain the megasporangio within which the meiosis occurs. 3 of the products of meiosis degenerate and the survivor develops in the only female spine or "megaspora" of the megasporangio, which unlike what happens in the other vascular plants, never leaves the megasporangio, is a distinctive feature of the sperm. The megaspora produces in its interior the female gametophyte, which in its adult state can be of few (6) or many cells, which never leaves the megaspora ("endosporic metophyte", common phenomenon in heterospore plants). The female gametophyte in turn produces in its interior one or more female gametes or "bones" (as in all embryos, the female gameta never leaves the female gametophyte). This new structure (megasporangio + megaspora + gametofito + gameta) is called egg.
- The cover of megasporangio for mitosis is forming the protective covers of the future embryo (called clutters, 1 in gymnospermas and 2 in angiospermas), there may be a part of the megasporangio that becomes a cover of reserve tissue (nucela).
- As in spermatophytes the megaspora never leaves the megasporangio, the megasporangio of sperm has a relatively specialised opening by natural selection to receive pollen grain and achieve fertilization. This megasporangio opening is called micropila. In gimnospermas the egg and its micrópila are exposed, in angiospermas they are covered by the carpelo (fertile sporang carrier leaf). The female gametophyte produces female gametes near the opening of the micropile.
- In the flower the fertile leaves carriers of microsporanges (the "microesporophiles") will be called stamina and will develop the microsporangio (which in the sperm stamens is called "polynic squash") that will give the microspores, each with a single male gametophyte (the mature male gametophyte covered by the wall of the microspora. Pollen grains will be released outside by "pollination" (opening of the polynic sac or microsporangio).
- in the flower the fertile leaves of megasporangios (the "megaesporophiles") will contain the megasporangio within which the meiosis occurs. 3 of the products of meiosis degenerate and the survivor develops in the only female spine or "megaspora" of the megasporangio, which unlike what happens in the other vascular plants, never leaves the megasporangio, is a distinctive feature of the sperm. The megaspora produces in its interior the female gametophyte, which in its adult state can be of few (6) or many cells, which never leaves the megaspora ("endosporic metophyte", common phenomenon in heterospore plants). The female gametophyte in turn produces in its interior one or more female gametes or "bones" (as in all embryos, the female gameta never leaves the female gametophyte). This new structure (megasporangio + megaspora + gametofito + gameta) is called egg.
- The new phenomenon of pollination which is the transport of pollen to the structures of the female flower prepared to receive it, through external agents. In gymnospermas the pollen is transported directly to the micropill, angiospermas is transported to the stigma of the carpel. External agents are mainly the wind (anemophilia phenomenon, common in gymnospermas) or animals (zoophilia phenomenon, common in angiospermas).
- When the pollination is successful, the male gametophyte grows, crosses the wall of the pollen grain and emits a Polynic tube o haustorial, which will emit polyflagelated anterozoids — primitive caracter — or the spermatic nuclei — advanced caracter — in the egg producing fertilization.
- After fecundation the female gametophyte by mitosis is forming a nutritious reserve tissue (haploid in gymnospermas, called prolo or primary endosperma, triploid in fertilization angiosperms with a spermatic nucleus of pollen, called endosperma by dry).
- As in all embryos, the zygote by mitosis becomes an embryo, which grew nourished by the female gametophyte through a placenta. In spermatophytes, the embryo when ripening enters into a state of latency surrounded by the structures of gametophyte —prolo or endosperma — and feminine sphinx — noose or no, 1 or 2 teguments — forming the seed.
- the embryo is bipolar with apical meristema and radical meristema.
- the mature seed is liberated by becoming the dispersion unit (unlike the pteridophytes spora).
- in appropriate conditions the germ seed (threatening of latency), the first phase of germination is the absorption of water and then the embryo is developed by mitosis, nourished at the beginning of the seed's reserve tissues and after its own products of photosynthesis, giving the new adult sporaphyte.
Evolution of spermatophytes
As can be seen from their living brethren the ferns, spermatophytes descend from an ancestor characterized by homospory (a single type of spore, always bisexual gametophytes). A critical step in seed development was the evolution of heterospory: the production of two types of spores, megaspores and microspores, which will give rise to male and female gametophytes, respectively.
Heterospory originated many times independently in unrelated lineages of vascular plants (there are examples in lycophytas, equisetopsids and polypodiopsids), in many of these cases the development of heterospory was followed by a reduction in the number of functional megaspores. In the line that led to seed plants, in the adult sporophyte that develops its megasporangia, meiosis of only one cell per megasporangia occurs, and by abortion of 3 of the products of meiosis, the number of functional megaspores is reduced. to only one functional megaspore per megasporangium. That single functional megaspore, in the spermatophyte lineage, was retained within the megasporangium, developing its female gametophyte and female gamete entirely within the megasporangium of the previous sporophytic generation. Finally, the megasporangium developed the integuments, leaving open the small hole called the micropyle.
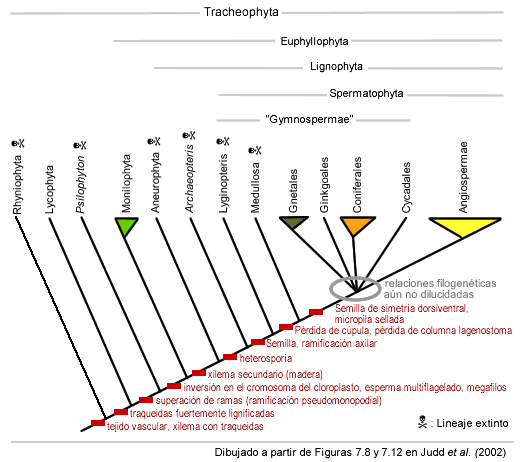
Our knowledge of the origins of the seed is based primarily on well-preserved fossils from the late Devonian and early Carboniferous, which were called "progymnosperms" or "seed ferns". Recall that the differentiation between a main stem and lateral branches had already evolved in the euphyllophyte lineage. The first thing that appears in the lineage that derived in the spermatophytes, was found in the late Devonian, and is the appearance of very large trunks, with wood quite similar in structure to that of modern conifers. These trunks were connected to huge branch systems bearing many small leaves. Archaeopteris, as it is now called, was discovered to be heterosporic, but not yet forming seeds.
The most accurate reconstruction and placement in the phylogenetic tree of Archaeopteris and other "progymnosperms" such as Aneurophyton (Beck 1981, 1988, Beck and Wight 1988), was instrumental in enabling scientists to establish both the origin of heterospory and wood production, and to conclude that both were prior to the evolution of the seed. It is therefore incorrect to say that these two fossils belong to a clade called spermatophytes ("seed plants"), even though they are ancestors of modern seed plants. Therefore the spermatophytes and those two fossils belong to a larger monophyletic group, which was called "Lignophyta" (Doyle and Donogue 1990), referring to the fact that they already produced wood.
Careful analyzes (eg Serbet and Rothwell 1992) have revealed that the first seeds were located in "domes" ("cupules" in English) and each seed was covered by an excrescence from the sporangium wall that formed a specialized chamber to receive pollen, or "pollen chamber". This structure was probably helped by a secretion of a sticky drop ("pollination drop") to capture the pollen grains.
The outer integument tissues probably derive from a series of sterile sporangia, which initially had the shape of a series of lobes at the seed apex, rather than the shape of a differentiated micropyle (see graph).
Phylogeny
Current Knowledge
Contrary to expectations, it has been shown that the group of living gymnosperms is a monophyletic group, sister to the angiosperms.
Molecular multigenetic analyzes have shown that Spermatophyta are divided into two large groups: Gymnospermae and Angiospermae, which were defined by John Ray in 1703, and taxonomically this classification has remained stable since 1854. Thus, the most recent phylogeny update (2014) of living seed plants presents the following subgroups:
| Spermatophyta |
| ||||||||||||||||||||||||||||||||||||||||||
The differences between the two groups are summarized in the following table.
| Structure | Gymnospermae | Angiospermae |
|---|---|---|
| Gametofito | With an archdiocese, or archgonio, or reduced, but not much. | Very small, only 3 cells the male and 7 cells with 8 nuclei the female. |
| Male Gametas | Mobile flags | There are no gametas, there are only spermatic cores. |
| Type of fertilization | Simple fertilization. By symphonogamy or zoidiogamy | Double fertilization (female gameta is fecundated forming a 2n zygote and a female gametophyte cell that will give endosperma 3n). Always symphony. |
| Ovul fabrics | Gameta feminine + protalo (metophyte feminine haploide converted into reserve tissue) + 1 tegumento from megasporangio | Gameta feminine + endosperma 3n + nucela and 2 teguments from megasporangio |
| Vascular fixture | Type tracheted | Tracheas |
| Flower | Unisexual | Hermaphrodite flowers fundamentally |
| Additional bracts of the flower | Bracteada flower, they have no perianto, they are acclaimed | Bracteada flower (with 1 or 2 prophylls) and peranto (composed by petals and sepals) with animal atractor function |
| Type of pollination | By anemophilia (transport agent is the wind) | Typically by zoophilia (transport agent are animals) |
| Dispersion unit | The seed: naked or at the most grouped in pseudofruits, the egg is exposed to the air. | The fruit (smile surrounded by the wall of the carpal) |
| Biotype | Trees or bushes with lignine | Any biotype |
| Type of branching | Monopodyal: All axes are open to growth | Syndicate: the axles are closing to growth. |
Extinct groups
An overview of extinct groups related to Spermatophyta would be as follows:
| Lignophyta |
| ||||||||||||||||||||||||
Historical overview
For much of the last century, seed plant lineages, both living and extinct, were commonly divided into two major groups: the cycadophytes and the coniferophytes. Cycadophytes, including modern Cycads, were distinguished by rather limited wood production (secondary stem growth), with rather broad rays (so-called "manoxylic log"), and by large fern frond-like leaves and radially symmetrical seeds. In contrast, in coniferophytes, including Ginkgo and conifers, the wood is well developed and dense ("pycnoxylic log"), the leaves are simple and often needle-shaped, and seeds dorsiventrally symmetrical (they are "squashed"). This distinction suggested to some researchers that seed plants actually originated twice. From this point of view, the cycadophyte line was derived from a Progymnosperma-like ancestor, in which the flattened lateral branching system derived into large fern-like leaves. Instead the spermatophyte line would have derived from an ancestor of the Archaeopteris type, in which individual leaves may have been modified into needle-like leaves. This scenario implies that the seed itself was originated twice, each corresponding to a different type of symmetry.
However, in phylogeny analyzes that included living lineages along with fossil representatives, they generally support the phylogenetic tree shown in the figure below (see for example Crane 1985, Doyle and Donoghue 1986, Nixon et al. 1994, Rothwell and Serbet 1994).
These studies assume that the seed appeared only once, and that the earliest seed plants were rather cycad-like, at least with respect to the large, pinnate leaves and radially symmetrical seeds. Specifically, it appears that a number of "seed ferns" from the Devonian-Carboniferous (Lygniopteris and medullosas) are situated at the base of seed plant phylogeny, and that the coniferophytes are nested several levels into the tree, in a clade " platyspermic" (from seeds with dorsiventral symmetry). This tree hypothesizes that the change to needle-like leaves and the change to dorsiventrally symmetrical seeds occurred after the appearance of leaves and seeds, and were probably an adaptation to arid-type environments.
Despite the enormous efforts made so far to elucidate the phylogenetic relationships of the 5 living groups of spermatophytes (cycads, ginkgos, conifers, gnetophytes, and angiosperms), using both morphological and molecular information, the relationships still do not receive consensus, and are the subject of various discussions in the scientific environment. In this sense, it should be clarified that it is definitively ruled out that angiosperms derive from an ancestral gnetophyte. Detailed morphological studies show a different origin of the xylem vessels in gnetophytes and in angiosperms (this means that they originated twice), and the flower structures of gnetaceae (here we define "flower" as & #34;defined growth branch bearing fertile leaves") are not homologous to those of the angiosperm flower. In addition, in 2004, fossils of Gnetophytes were found that confirm (morphologically) their belonging to the group of gymnosperms, and once again rule out their relationship with angiosperms.
Keep in mind that it was also grouped within the "gymnosperms" to many fossils of extinct seed plants (the pteridosperms) which together do not form a monophyletic group: in fact the group called Gymnosperma sensu lato becomes paraphyletic when one takes into consideration basal spermatophyte lineages, as well as other "seed ferns" from the late Permian and Mesozoic, some of which are probably in the lineage that led to angiosperms.
Spermatophyte systematics
Classification
Different classification systems give different relative importance to spermatophyte structures:
According to the Engler classification system, they are subdivided into:
- Division Embryophyta siphonogama (=Spermatophyta)
- Gymnospermae Branch (Cycadofilics, Cycadales, Bennettitals, Ginkgoales, Coniferae, Cordaitals, Gnetales)
- Angiospermae Branch
- Class Monocotyledoneae
- Class Dicotyledoneae
According to the Cronquist system they are divided into:
- Progimnospermas (progimnospermophytina subdivision).
- Cicadofitinos (Cycadice subdivision, Cycadophytina is a synonym) or gimnospermas of pine leaf.
- Pteridospermas or liginoptéridas (Pteridospermopsida class).
- Benetitatas (Cycadeoidopsida class).
- Cycadatas (class Cycadatae, Cycadopsida is a synonym).
- Coniferofitinos (Pinicae subdivision, Coniferophytina is a synonym) or gimnospermas de dicótoma sheet.
- Ginkgoatas (class Gincoatae).
- Pinatas (class Pinatae, Coniferopsida is a synonym).
- Gnetofitinos (Gneticae subdivision, Gnetophytina is a synonym).
- Gnetatas or clamidospermas (Gnetoatae class, Gnetopsida is a synonym).
- Angiospermas (subdivision Magnoliophytina).
- Dicotylenes (class Magnoliopsida).
- Monocotlers (Lilopsida class).
Spermatophyte diversity: living groups
There are two living lineages of spermatophytes today: gymnosperms and angiosperms. Gymnospermae refers to the fact that they have "naked" or not completely covered by the carpel, as opposed to angiosperms or flowering plants, whose carpel completely covers the seed.
Gymnosperms
Cycads
They were the most abundant and diverse during the Mesozoic. Today about 130 species remain. Cycads generally have a low, broad trunk, limited secondary xylem, and large, fern- or palm-like compound leaves. They are dioecious, which means that some sporophytes only carry ovules and then seeds, and other sporophytes only carry pollen-producing male strobili. Both types of strobila are typically very large, and in some cases brightly colored. Likewise, the seeds are usually large and usually have a fleshy and colored external integument, presumably an adaptation to attract vertebrate dispersal agents. Many cycad features may be ancestral, such as haustorial tube pollen (instead of pollen tube), and multiflagellate giant sperm (instead of sperm nuclei). However, cycads possess unique characteristics that set them apart from ancestral seed plants, which are presumably derived traits, including loss of axillary branching, presence of girdling leaf-patterns; ("with girdles?"), and the production of coralloid roots that harbor nitrogen-fixing cyanobacteria. Within the cycads, phylogenetic analyzes indicate that the first division of the group was the one that divided the lineage of Cycas from the rest. Therefore Cycas would be retaining some presumably ancestral characters, such as those found in fossil relatives such as Taeniopteris, namely: having many ovules born on the margins of carpels (carpels defined as ovule-bearing fertile leaves) with foliar-type morphology, instead of having two ovules per peltate carpel that supports them pointing towards the axis of the sporophyll (on its "adaxial face"), which is the character found on the other line. Also in Cycas the fertile leaves bearing ovules are not grouped in strobili, as they are in the other line.
Ginkgoes
There is only one surviving species of ginkgo: Ginkgo biloba. This species is very rarely found in the wild, but the trees present in the temples of China were maintained for centuries by the monks who inhabit them, and in modern times it was cultivated by man on the sidewalks of the cities. Perhaps the most distinctive feature of modern Ginkgo is the production of deciduous, fan-shaped leaves ("flabellate"), with dichotomous venation. Ginkgos are well known from the fossil record, where great diversity in leaf morphology is observed. Like cycads, ginkgos are dioecious (different sporophytes bear either carpels or stamens—stamens defined as "fertile leaves bearing pollen sacs that contain pollen grains"). The ovules are borne in pairs on axillary branches which are thought to be reduced strobila. The ovule integument differentiates into a fleshy (and odorous) outer layer and a stony (hard) inner layer that encloses the female gametophyte. Also like cycads, ginkgos retain several characters from ancestral spermatophytes, such as pollen emitting a haustorial (non-pollinal) tube, and flagellate sperm capable of swimming.
Conifers
There are about 600 species of living conifers. They are trees or shrubs with well-developed wood and usually needle-like leaves. Normally the leaves are solitary, growing along the stem, but in pines (Pinus) they are grouped on small twigs. Leaves usually have additional adaptations to dryness, eg sunken stomata. However, some Southern Hemisphere conifers (eg Podocarpus, Agathis) have large, flattened leaves, and Phyllocladus have flattened branches that they look like leaves. Many conifers are monoecious (they have carpels and stamens on the same sporophyte), but some groups are dioecious: Juniperus, Taxus, and Podocarpus. In the male strobili (which in conifers are called male cones or "pollen cones" in English), the stamens (or "microsporophylls") support the sporangia (& #34;microsporangia") that will give male gametophytes ("microgametophytes"). Pollen grains are the male gametophytes protected by a wall originating from the sporophyte, and sometimes have a pair of sac-like air-filled appendages, presumably adaptations to wind dispersal, but these 'air sacs'; they seem to have been lost in many lines. The receptive ovules, unlike the microsporangia, are located on the adaxial face of each carpel or "ovuliferous scale", facing the axis of the female cone. The meiosis that will give the gametes, occurs within each ovule, and only one of the 4 products of meiosis will develop until giving the female gametophyte, always within the ovule. The female gametophyte, or "protalo", produces one or more female gametes or "eggs" in the sector close to the micropile. When the pollen grain finally reaches the micropyle, the enclosed male gametophyte develops a tube ('pollen tube') which passes through the wall of the female gametophyte. When the pollen tube finishes growing, the male gametophyte emits two sperm through it, which can be cells or cell nuclei ("sperm nuclei") depending on the lineage. The phenomenon of "polyembryony" is very common in conifers, with many embryos developing in the same female gametophyte, which may be due to independent fertilization events in which several eggs were fertilized by several tubes. different pollen types, or because the single embryo split at an early stage into several genetically identical embryos, the latter possibility being more commonly encountered than the former. In modern conifers, the pollen-bearing strobila is said to be 'simple', while the ovule-bearing one is said to be 'compound'. This is due to how the morphology of the strobila is interpreted: The male cone is interpreted as a modified branch carrying fertile leaves or stamens, therefore it would be a "flower" sole bearer of many stamens. On the other hand, the female cone is interpreted as derived from a branch with leaves, which in turn carry lateral branches of defined growth born in the axil of the leaves, each of the lateral branches carrying fertile leaves or carpels. This interpretation is supported by the fossil record, which shows a series of steps in the reduction of the carpel-bearing lateral branches, until the appearance of the "ovuliferous scale" highly modified that we see in modern groups (Florin 1951, 1954). It is also observed that each ovuliferous scale is supported by a bract ("tector bract"), which would represent the leaf that bears the lateral branch, also highly modified. In a few conifers, the bract is conspicuous, emerging from between the ovuliferous scales (for example in Pseudotsuga mensiezii). However, in many conifers the bract is extremely small. In Cupressaceae Taxodium and Cryptomeria, the bract is fused to the ovuliferous scale, and the ovuliferous scale still shows signs of "leafing" (visible as small teeth). Phylogenetic studies have revealed some interesting questions about the evolution of conifers. (eg Stefanovic et al. 1998). Molecular data show a basal split between Pinaceae and a clade that would house all other conifers. Pinaceae have several unique characteristics, such as inverted ovules (with the micropyle facing the axis of the cone) and winged seeds, wings that originate from the ovuliferous scale during seed development. Within the other clade of conifers, the two largest groups in the Southern Hemisphere (Podocarpaceae and Araucariaceae) form a clade, presumably with the synapomorphy of having only one ovule per ovuliferous scale.ra. The Cupressaceae are marked by many unique characteristics, such as the fusion of the ovuliferous scale with the tectriz bacteria. In turn, this group may be related to the Taxaceae, which have highly reduced female cones with a single terminal seed, surrounded by a fleshy and colorful third integument ("aryl").
Gnetals
This group contains only about 80 living species, belonging to three quite distinct lineages (Doyle 1996, Friedman 1996, Price 1996). One is Ephedra, with about 40 species distributed in deserts around the world, with very small scaly leaves. Another is Gnetum, with about 35 species in Old and New World tropical forests, with entire-lamina leaves much like those seen in most angiosperms. Finally, Welwitschia with a single species, Welwitschia mirabilis found in southwestern Africa, produces only two or rarely four functional leaves throughout its entire life, leaves that grow indefinitely by meristems present at the base, gradually becoming necrotic at the tips.
Although these three clades look very different from one another, they share many unusual characteristics, such as opposite leaves, multiple buds per axil, xylem vessels with circular openings between attached cells, compound pollen, strobilous seeds, and a pollen ancestral ellipsoid with characteristic striations running from end to end. The seeds also have two integuments, the inner one forming the micropylar tube that the pollination drop exudes, the outer one derived from a pair of fused bracts. Molecular studies also highly agree on the monophyly of this group. Within the gnetophytes, Gnetum and Welwitschia form a well-consensus clade. Some of the morphological synapomorphies are: leaves with reticulate venation, further reduction of the male gametophyte, and some aspects of the structure of the female gametophyte, such as tetrasporic development, loss of archegonia, free nuclei functioning as eggs instead of the cells. The characteristic striated pollen found on Ephedra and Welwitschia was apparently lost in the line from which it derived Gnetum, which has pollen with shaped granules. peak, not open. As far as the fossil record is concerned, it is rather poor except for pollen grains. Only a few macrofossils have been described (Crane 1996). Although the gnetophyte pollen grains are found from the Triassic, it seems that the clade containing the modern groups has diversified more significantly during the middle Cretaceous, at the same time as the angiosperms. Like angiosperms, gnetophytes shortened their life cycle (and probably became herbaceous) and evolved alongside insects to be pollinated by them, a feature still found in some living groups. In stark contrast to angiosperms, on the other hand, gnetophytes never became a significant component of the flora at high and mid-paleolatitudes, and have suffered a dramatic decline in their representativeness during the late Cretaceous (Crane et al. 1995, Crane 1996).
Angiosperms
With some 257,000 living species, angiosperms are responsible for most of the diversity in spermatophytes, embryophytes, and viridophytes. Strong evidence for angiosperm monophyly comes from molecular studies and from the many morphological characters shared by members of this clade. Of these, some of the most obvious, which are also important reproductive characteristics, are: (1) the seeds are produced within a carpel with a stigmatic surface that allows pollen germination, (2) the female gametophyte is very small, in most species there are only 8 nuclei in 7 cells, and (3) double fertilization, which led to the formation of a characteristic triploid nutritive tissue called the endosperm. Other characteristics are: (4) many angiosperms have xylem vessels instead of tracheids, a derived character within the group, water can flow in the vessels without having to cross a membrane, which makes them very efficient in transporting fluids within the sporophyte, but probably also more prone to damage (especially by air emboli) when subjected to water stress. (5) The phloem of angiosperms differs from that of all other plants in that the elements of the sieve tube (which are living cells, but without a nucleus, responsible for the transport of sugars) are accompanied by one or more "companion cells& #34;, which are born from the same mother cell as the sieve element.
Contenido relacionado
Genetic fingerprint
Albufera Natural Park
Open