Sodium chloride
Sodium chloride, common salt or table salt, known in its mineral form as halite, is a chemical compound with the NaCl formula. Sodium chloride is one of the salts responsible for the salinity of the ocean and the extracellular fluid of many organisms. It is also the component of common salt, used as a seasoning and food preservative.
With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g of Na and 60.66 g of Cl. In its edible form, salt (also known as table salt) is commonly used as a seasoning and as a food curing and preservative Large amounts of sodium chloride are used in many industrial processes, and it is an important source of sodium and chlorine compounds used as raw materials for further synthesis chemicals. Another important application of sodium chloride is the de-icing of roads when the weather is below zero.
In antiquity, sodium chloride was highly sought after as a tradable good and as a condiment, and in Roman preclassic times it was paid to the soldiers who built the Via Salaria, which began in the quarries from Ostia to Rome, with a generous salarium argentum.
Chemical properties
It is an ionic compound formed by a sodium cation (Na+) and a chloride anion (Cl-), and it can undergo the characteristic reactions of either of these two ions. Like any other soluble ionic chloride, it precipitates insoluble chlorides when added to a solution of an appropriate metal salt, such as silver nitrate:
- NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
Another method to separate both components is through electrolysis.
If a direct electric current with a high potential is applied to an alkaline brine, the anodic product is dichlorine gas (Cl2) and the cathodic ones are sodium hydroxide (NaOH) and dihydrogen (H 2).
- electrolysis: 2 NaCl(aq) → 2 NaOH(aq) + Cl2(gas) + H2(gas)
Like most ionic salts, it confers colligative properties to its solutions, that is, it is capable of varying the vapor pressure of the solution, raising the boiling point and lowering the freezing point according to its molal concentration.
Crystal structure
When sodium chloride is in the solid state, its atoms are arranged in a cubic crystalline structure, as would be expected in an ionic bond caused by the electrostatic fields of its atoms. Each ion fits in the center of a regular octahedron, being surrounded by 6 oppositely charged ions distributed at the vertices of the octahedron.
This same basic structure is found in many other compounds and is commonly known as the halite or rock salt crystal structure. It can be represented as a face-centered cubic (fcc) lattice with a basis of two atoms or as two interpenetrating face-centered cubic lattices. The first atom is found at each lattice point.
Production
Sodium chloride is produced en masse by evaporating seawater or brine from other resources, such as salt lakes, and mining rock salt, called halite.
In 2002, world salt production was estimated at 210 million metric tons, and the main producing countries were the United States (40.3 million tons), China (32.9), Germany (17.7), India (14.5) and Canada (12.3). A
Physiological regulation
The Na+ ion is the cause of cellular osmotic regulation, regulating the membrane potential by expelling the K + ion, greatly facilitates the nerve impulse and is contributed to the organism largely as table salt.
Applications
Sodium chloride is universally used as a food additive. It is also used in the production of paper and cellulose, in bathroom products and in detergents. In addition to the familiar household uses of salt, the most dominant applications of the production of about 250 million tons per year (2008 data) include chemicals and de-icing.
Local antiseptic and food preservative
Salt, thanks to its high osmotic power, is capable of dehydrating a wide spectrum of viruses and bacteria in a non-spored state, which is why it is used as a painful antiseptic to disinfect wounds.
Very few microorganisms, such as halophiles and higher organisms such as brachiopod crustaceans known as Artemia, can resist the osmotic power of salt. Among the microorganisms resistant to salinity is the case of the bacterium B. marismortui, found in the Dead Sea.
Salting meat is a way to preserve it from bacterial action, since salt delays protein breakdown. To preserve food, sodium chloride extracts water from it, preventing the growth of bacteria.
Diverse industrial uses
Sodium chloride is widely used, so even relatively minor applications can consume massive amounts. In oil and gas exploration, salt is an important component of drilling fluids in the drilling of wells. It is used to flocularize and thicken drilling fluid to overcome high downhole gas pressures. Whenever a drill strikes a salt formation, salt is added to the drilling fluid to saturate the solution in order to minimize dissolution within the salt layer.Salt is also used to increase the cure of concrete in cemented casings. >.
In textiles and dry cleaning, salt is used as a brine rinse to separate organic contaminants, to promote "salting" of dye precipitates and to mix with concentrated dyes to standardize them. One of its main functions is to provide the positive ionic charge to promote the absorption of negatively charged ions from dyes.
It is also used in the processing of aluminum, beryllium, copper, steel and vanadium. In the pulp and paper industry, salt is used to bleach wood pulp. It is also used to make sodium chlorate, which is added along with sulfuric acid and water to make chlorine dioxide, an excellent oxygen-based bleaching chemical. The chlorine dioxide process, which originated in Germany after World War I, is becoming increasingly popular due to environmental pressures to reduce or eliminate chlorine bleach compounds. In tanning and leather treatment, salt is added to animal hides to inhibit microbial activity on the underside of the hides and to draw moisture back into the hides.
In rubber manufacturing, salt is used to make buna, neoprene, and white rubber. The brine and sulfuric acid are used to coagulate an emulsified latex made from chlorinated butadiene.
Salt is also added to secure the soil and firm the foundations on which highways are built. The salt acts to minimize the effects of subsurface displacement caused by changes in humidity and traffic loading.
Sodium chloride is sometimes used as a cheap and safe desiccant due to its hygroscopic properties, making salting an effective method of food preservation historically; salt draws water from bacteria through osmotic pressure, preventing them from reproducing, one of the main sources of food spoilage. Although more effective desiccants exist, few are safe for human ingestion.
Ice Flux
Sodium chloride in brine (the commercial mixture for a saturated solution reaches 270 g per liter) is the most widely used flux for ice and snow on roads. As salt dissolves in water, it lowers the freezing point of the water. In contact with water, it causes an endothermic reaction that requires heat input, which it takes from the environment or from the contact surface, enthalpy, (ΔH= -385.820 KJ/mol).
The second most important application of salt is de-icing and anti-icing roads, both in sand deposits and spread by winter service vehicles. In anticipation of snowfall, the roads are "anti-iced" optimally with brine (concentrated solution of salt in water), which prevents adhesion between snow and the road surface. This procedure avoids the intensive use of salt after snowfall. For deicing, brine and salt mixtures are used, sometimes with additional agents such as calcium chloride and/or magnesium chloride. The use of salt or brine becomes ineffective below −10 degrees Celsius (14 °F).
Deicing salt in the UK comes predominantly from a single mine at Winsford in Cheshire. Prior to distribution, it is mixed with <100 ppm sodium ferrocyanide as an anti-caking agent, allowing the rock salt to flow freely out of blast vehicles despite being stored prior to use. In recent years, this additive has also been used in table salt. Other additives have been used in road salt to reduce overall costs. In the US, for example, a carbohydrate solution by-product of sugar beet processing was mixed with rock salt and adhered to road surfaces 40% better than loose rock salt. By staying on the road longer, the treatment did not have to be repeated multiple times, saving time and money.
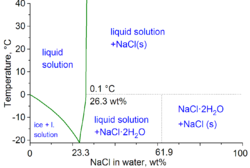
In technical terms of physicochemistry, the minimum freezing point of a mixture of water and salt is −21.12 degrees Celsius (−6.0 °F) for 23.31% by weight of salt. However, freezing near this concentration is so slow that the eutectic point of −22.4 degrees Celsius (−8.3 °F) can be reached with 25% by weight of salt.
Environmental effects
Road salt ends up in freshwater bodies and could harm aquatic plants and animals by disrupting their ability to osmoregulate. The ubiquitous presence of salt poses a problem in any shoreline coating application, as trapped salts cause major adhesion problems. Naval authorities and shipbuilders monitor salt concentrations on surfaces during construction. Maximum salt concentrations on surfaces are authority and application dependent. The IMO regulations are the most widely used and set salt levels at a maximum of 50 mg/m2 of soluble salts measured as sodium chloride. These measurements are made using a Bresle test. Salinization (increased salinity, also known as freshwater salinization syndrome) and the consequent increase in metal leaching is an ongoing problem throughout North America and European fresh waterways..
In highway melting, salt has been associated with corrosion of bridge decks, motor vehicles, reinforcing bars and wires, and unprotected steel structures used in highway construction. Surface runoff, vehicle spray, and wind actions also affect soil, roadside vegetation, and local surface and groundwater supplies. Although evidence of environmental loading of salt has been found during peak use, spring rains and snowmelt often dilute sodium concentrations in the area where salt was applied. A 2009 study found that approximately 70% of salt of highway that is enforced in the Minneapolis-Saint Paul metropolitan area is retained in the local watershed.
Plants
Sodium is a non-essential nutrient for plants, which need it in very low doses. Exceptionally, certain groups of plants, such as C4 plants, or CAM, require higher doses of this element.
On the other hand, excess salt in the medium is detrimental to most plants (salinity of the soil or substrate), since it causes its drying by osmosis (water moves towards the more saline environment). A characteristic symptom is burns on the edges of the leaves. Only plants called halophytes have developed a process that prevents this loss of water.
Food industry and agriculture
Many microorganisms cannot live in a salty environment: the water leaves their cells by osmosis. For this reason, salt is used to preserve some foods, such as bacon, fish, or cabbage.
Salt is added to foods, either by the producer or by the consumer, as a flavor enhancer, preservative, binder, fermentation control additive, texture control agent, and color developer. Salt consumption in the food industry is subdivided, in descending order of consumption, into Other Food Processing Products, Meat Packers, Canning, Bakery, Dairy Products, and Grain Milling. Salt is added to promote color development in bacon, ham, and other processed meat products. As a preservative, salt inhibits the growth of bacteria. The salt acts as a binder in sausages to form a binding gel made up of meat, fat and moisture. Salt also acts as a flavor enhancer and softener.
In many dairies, salt is added to cheese as an agent to control color, fermentation, and texture. The dairy subsector includes companies that manufacture cream butter, condensed and evaporated milk, frozen desserts, ice cream, natural and processed cheese, and specialty dairy products. In canning, salt is added primarily as a flavor enhancer and preservative. It is also used as a carrier for other ingredients, a dehydrating agent, an enzyme inhibitor, and a softener. In bakery, salt is added to control the rate of fermentation of the bread dough. It is also used to strengthen gluten (the elastic protein-water complex of certain doughs) and as a flavor enhancer, for example as a coating for baked goods. The food processing category also includes milling products. These products consist of milling flour and rice and manufacturing breakfast cereals and blended or prepared flour. Salt is also used as a seasoning, for example in chips, pretzels, and cat and dog food.
Sodium chloride is used in veterinary medicine as an emesis-causing agent. It is administered as a warm saturated solution. Emesis can also be caused by the placement of small amounts of common salt or salt crystals in the pharynx.
Medicine
It is the natural antidote to silver nitrate, metabolizing it into silver chloride, a practically non-toxic substance that the body can safely excrete.
Contenido relacionado
William Hyde Wollaston
Gunpowder
Surface tension