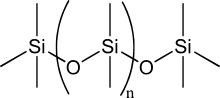
Silicone
Silicone is an inorganic polymer derived from polysiloxane, made up of a series of alternating silicon and oxygen atoms. It is odorless and colorless. Silicone is inert and stable at high temperatures, which makes it useful in a wide variety of industrial applications, such as lubricants, adhesives, molds, and in medical and surgical applications, such as valve and cardiac prostheses, and breast implants. It can be sterilized by ethylene oxide, radiation, and autoclave processes. They constitute the most important branch of organosilicon derivatives; The essential characteristic of polymers is to present in their molecule, in addition to the silicon-carbon bond, the silicon-oxygen bond, which gives rise to their name: silicones.
In addition, silicone is the name given to the family of chemical compounds synthesized for the first time in 1938.
History
In 1824, the Swedish chemist Jöns Jacob Berzelius, who was based on the hypothesis of the French chemist Antoine Lavoisier regarding metal oxides and took advantage of the works of the also English chemist Humphry Davy, demonstrated the silica structure, isolating the silicon. Berzelius thus obtained the silicon tetrachloride SiCl
4 and the German Friedrich Wöhler the "silicio-chloroform" SiHCl
3 (triclorosilano); the Frenchman Charles Friedel and the American James Crafts prepared the first compounds of silicon-carbon by reaction of zinc dietilo between 1863 and 1866. Zn(C
2H
5)
2 about silicon tetrachloride. In 1872 the German Albert Ladenburg was the first to observe the formation of a hydrolysed polysiloxan fluid, the diethyyldietoxysilicio (CDC)2H5)2 Yes (OC)2H5) 2 ⋅ ⋅ {displaystyle {ce {ce {cHFFFF}}
However, interest waned until silicone polymers were discovered at the beginning of the 20th century by the English chemist Frederic Stanley Kipping, who is considered a pioneer in the subject. He was looking for stereoisomeric elements, in order to obtain one that would be compatible with hydraulic fluids, synthetic rubber, and also capable of repelling water. He wanted the war industry to be useful that they were in times of war, since he wanted to find a way to make the equipment less heavy and improve the lubricants for the machines, the cars and the shoes of the soldiers who were on the combat front. Since 1903, Kipping worked to find new techniques for the synthesis of a variety of organic compounds based on silicon. He called these new substances silicones and at first they were used to lubricate, especially during World War II. They began to be obtained industrially from 1930.
Frederic Kipping and Matt Saunders coined the word silicone in 1901 to describe polydiphenylsiloxane by analogy to their formula, Ph
2SiO (Ph stands for phenyl, C
6H
5), with the formula for the ketone benzophenone, Ph
2CO (originally named silicoceton to). Kipping was well aware that polydiphenylsiloxane was polymeric while benzophenone was monomeric and noted that the Ph
2SiO and the Ph
2CO had very different chemistries. The discovery of structural differences between the Kipping molecules and ketones meant that silicone was no longer the correct term (although it is still in common use) and that the term siloxanes is correct according to modern chemistry nomenclature.
Trying to combine the properties of carbon compounds with silicon compounds, a Corning Inc. (USA) researcher, James Franklin Hyde, invented silicones in 1938. The Dow Corning company was founded in 1943 to exploit the invention that led to a large number of resins, varnishes, elastomers and other human uses, the use of which Kipping could never have imagined. In 1949, when Kipping died, he had already published about 51 articles on the subject.
Taking Kipping as a pioneer, the Plastic Historical Society was created in 1986, to draw attention to the heritage of the plastics industry, encourage the study of other polymers, including synthetics, rubbers and elastomers.
Origin
The primary product for the elaboration of silicones is silica (silicon dioxide), quite abundant in sandstone, beach sand and other similar rocks; Silica is also the main ingredient in glass. Silicones are made from chlorosilanes, tetraethoxysilane, and other similar silicon compounds. Depending on the conditions of its obtaining and subsequent chemical processes, its usefulness will be different.
Features
- Resistant at extreme temperatures (–60 to 250 °C)
- Resistant to weather, ozone, radiation and moisture
- Good resistance to fire
- Excellent electrical properties like insulation
- Great resistance to compression deformation
- Suitable for food and health
- He has the power to extend
- Permeability to gas
- Long service life
- Capacity to repel water and form seals, although silicones are not hydrophobic
Classification
Silicones can be classified in relation to the length of their molecules, which influences their use:
- Slots: less than 10 basic units
- Oils: between 10 and 100 basic units
- Resins: between 100 and 500 basic units
- Gomas: between 500 and 2000 basic units
Properties
Due to its chemical composition of silicon and oxygen, it is flexible and soft to the touch, it does not stain, it does not wear out, it does not age, it is resistant to the use that is given to it, it does not contaminate, and it can take shapes and show off colors, it has a low thermal conductivity, and a low chemical reactivity, it is not compatible with microbiological growth, it is not toxic, it has resistance to oxygen, ultraviolet radiation and ozone, it is highly permeable to gases at its ambient temperature of 25°C.
Mechanical properties
Tensile strength of 70 kg/cm² with an average elongation of 400%. It maintains these values even after long exposures to extreme temperatures.
Electrical properties
It is flexible, elastic and insulating, maintaining its properties at extreme temperatures where other materials would not withstand.
Biocompatibility
It is formulated with the FDA Biocompatiblity Guidelines for medicinal products. It is odorless, tasteless and does not develop bacteria, it is not corrosive with other materials.
Chemical resistance
They behave well in contact with most chemical agents, but they are attacked by greases and solvents. Silicone is resistant to some chemicals, including some acids, chemical oxidizers, ammonia, and isopropyl alcohol. Silicone swells when exposed to nonpolar solvents like benzene and toluene.
Silicone Grades
Fluids
Its molecule is made up of a chain of silicon and oxygen atoms. The most common fluids are called PDMS (polydimethylsiloxanes). Its main uses are:
- Refrigeration
- Dieléctricos
- Pigs and polishing products
- Antiespumante
- Preparation of cosmetic, pharmaceutical and medical products
There are different compounds derived from fluids:
- Emulsions
- Pastas and fats
- Antispum
Elastomers
They are formulated from linear reactive chains to which a cross-linking agent and mineral fillers are added to provide good mechanical properties. Its main uses are:
- Sealed seals
- Cable insulation
- Driving of hot gases and liquids
- Surgical prosthesis and appliances
- Sealing compounds
Resins
Being resins, they are relatively low molecular weight polymers that have a three-dimensional structure. Its main uses are:
- Insulating varnishes
- Industrial paints
- Encapsulation and impregnation agents
Chemical composition
Silicone polymers are produced by intermolecular condensation of silanols, which are obtained by hydrolysis of silicon halides. Silicon atoms can join one or more organic substituents, creating compounds with various properties and applications.
Different siloxane structures can be obtained depending on the R group. There are two types of silicones in relation to their composition and their polymerization method:
- Condensing polymerization
- Polymerization by addition
The main difference between the two types is that during condensation polymerization by-products (alcohol) are formed while addition polymerization does not.
In addition, addition silicones, since they do not form by-products, become the most dimensionally stable elastomer, but their cost is higher compared to the others due to the presence of platinum or palladium in their composition, to obtain a better impression and positive.
Condensation silicones
They are condensed, since ethanol is obtained as a by-product.
Composition
- Polymer: Polydymethyl siloxane with hydroxyl terminal group
- Activator: Tin octane
- Filling material: Colloidal silica
- Chain Crossing Agent: Alkylic Acid
- Plastificante: Dibutil ftalato
- Colorful
2 and 4 catalysts
1 and 3 bases
Presentation and proportion
- Pote base (heavy silicone)
- Tube activator
- Fluid silicone tube
A tablespoon of base + a diameter of activator → take an impression → with fluid silicone in a syringe, on top of the base, take an impression.
Advantages
- No unpleasant taste or smell
- Excellent deformation recovery
- Resistant to tear
- Working time and adjustable polymerization
- Relatively economic
- Clean work
Disadvantages
- Low dimensional stability
- It requires almost immediate emptied (20-30 min) to recover elastic memory and harden
- Hydrophobic
- Requires manual spacing
- Short expiration time
Additional silicones
They are additive because they do not release a by-product as such, there is only the release of H2, which does not affect the contraction, only the emptying.
Composition
- Polymer → Polyvinil siloxano.
- Activator (catalizer) → Chloroplatinic acid.
- Filling material → Colloidal silica.
- Intercruising agent of chains → Silano.
- Plastificante → Dibutil ftalato.
- Coloring.
Presentation and proportion
- Pote → base
- Tube → catalyst
- Tube → fluent silicone
- Can be presented on a self-mixing device
- Mix the base and catalyst in equal quantities
Advantages
- They are the most accurate available
- Pleasant smell and flavor
- Excellent dimensional stability
- High recovery to deformation
- Stables to disinfection
- Available in self-mixing devices → when using automixing devices we avoid bubble formation, as the proportions are accurate
- Hydrophilics
- Empty after 1 hour, at least → which the H2 is released and does not alter the emptied in the plaster
Disadvantages
- Cars
- Sensible to contaminant
- Release of hydrogen (volatile) during polymerization, emptying should not be done immediately (1 –24 h)
In Summary
| Advantages | Disadvantages | |
|---|---|---|
| They are very accurate, with great reproduction of detail, elasticity and appropriate resistance. In addition, its economic cost is low, in the case of condensation. | If polymerized by misunderstanding dimensional stability. His life in storage is short. | |
| If polymerized by addition, excellent dimensional stability, color, smell and pleasant taste, they do not require individual or adhesive portaimpression. | The high hydrophobicity of silicone makes it difficult to make an impression in a wet environment and also to obtain a positive plaster free bubble stone. |
Uses and applications
Due to its versatility, it has been used successfully in multiple daily consumer products. Such is the case of hairsprays, lipsticks, sunscreens and moisturizers. Given its low reactivity, it has been widely used in the pharmaceutical industry in the manufacture of capsules to facilitate the ingestion of some drugs, in antacids under the designation of methicone. There are over 1,000 medical products in which silicone is a component. It is also a substance commonly used as a lubricant on the inner surface of syringes and bottles for the preservation of blood derivatives and intravenous medications. Pacemakers, heart valves, and the Norplant all use silicone coatings. Implantable devices such as artificial joints (knees, hips), catheters for chemotherapy or hydrocephalus, drainage systems, implants are also manufactured with silicone.
Another application is silicone for molds as an alternative to latex in the manufacture of molds due to its flexible and non-stick properties.
The following groups can be organized:
Kitchen utensils
- Silicone can be used when a contact with food is required, it is not toxic and is inodorant.
- It is used in baking utensils and kitchen utensils.
- It is used as insulation in grippers, heat resistant
- It is used in chocolate, ice, cookies,
Automotive industry
Silicone grease is typically used as a lubricant for brake components, spark plug wires are insulated by multiple layers of silicone, silicone compounds are used as coatings and sealants for air bags.
Covering
Silicone film can be applied to silica-based substrates such as glass to form a covalently bonded hydrophobic coating.
Aquarium Joints
They use it as a sealant, to join glass plates.
Defoamer
Silicones are used as an active compound in defoamers due to their low water solubility and good spreading properties.
Dry clean
Liquid silicone can be used as a dry cleaning solvent, providing an environmentally friendly alternative.
Electronics
Electronic components are sometimes wrapped in silicone to increase stability against mechanical and electrical shock, radiation, and vibration.
Lubricants
Silicone greases are used in bicycle chains. There are also silicone personal lubricants for use in medical procedures or sexual activity.
Medicine
The gel form is used in bandages and dressings, breast implants, testicular implants, pectoral implants, contact lenses, and a variety of other medical uses.
Gynecology
In ecological alternatives for menstruation such as menstrual cups.
Ophthalmology
Silicone is used in intraocular lenses, after cataract extraction.
Plumbing and Building Construction
In the construction industry there are silicone caulks and sealants. In plumbing, silicone grease is applied to taps and valves, preventing limescale from adhering to metal.
Toys
High-bounce silicone balls are a common toy, coming in many shapes and variations.
There are also 100% organic food silicone teethers for babies, free of BPA, PVC and phthalate.
Safety and environmental considerations
According to Ullmann's Encyclopedia of Industrial Chemistry (Ullmann's Enciclopedia de Química Industrial, in Spanish), silicones have been observed to have "notable harmful effects on organisms of the environment". Although they are biodegradable, they are absorbed by solids in wastewater treatment facilities. Degradation is catalyzed by various clays.
Silicone is not a natural polymer, so it decomposes much longer than polymers made from corn, wheat or potato. A case for biodegradability can be seen with the silicone thimbles of some early space suits.
Production and marketing
Global demand for silicones approached $12.5 billion in 2008, up 4% from the previous year. It continued to have a similar growth in the following years until reaching USD 13.5 billion in 2010.
The world's leading manufacturers of silicone-based materials belong to three regional organizations: the European Silicone Center (ESC) in Brussels, Belgium; the Environmental Health and Safety Council (SEHSC) in Herndon, Virginia, USA; and the Silicone Industry Association of Japan (SIAJ) in Tokyo. The World Silicone Council (GSC) acts as an umbrella structure over regional organizations. Its main mission is to promote the safety of silicones from a health, safety and environmental perspective.
Warning
In English, the term for silicone is silicone, and should not be confused with silicon, which means silicon, a crystalline metalloid that is the base of silicone and widely used in the manufacture of electronic devices.
Contenido relacionado
Crystallography
Ligand
Princess of Asturias Award for Scientific and Technical Research
Polyoxometalate
Miller and Urey experiment