Saponification
Saponification is the name given to the chemical process of hydrolysis of an ester in a basic medium, by which a fatty body, united to a base and water, results in soap and glycerin. Soaps are called sodium and potassium salts derived from fatty acids. All those substances that contain traces of fatty acids in their molecular structure are susceptible to saponification, and are natural substances that we call saponifiable lipids. The most abundant saponifiable lipids in nature are neutral fats or glycerides. The saponification of a diglyceride is summarized as follows:
- soap + glycerin
This chemical process is used as a parameter to measure the composition and quality of the fatty acids present in oils and fats of animal or vegetable origin, this analysis being called the Saponification Index; which is a measurement method to calculate the average molecular weight of all fatty acids present. Likewise, this parameter is used to determine the percentage in the fatty bodies of unsaponifiable materials, that is, substances that do not contain fatty acids.
A common industrial saponification method involves boiling the fat in large kettles, slowly adding sodium hydroxide (NaOH), and stirring continuously until the mixture begins to slurry.
History
Initially, this reaction was known to transform the mixture of a glycerol ester and a strong base into a mixture of soaps (or salts of fatty acids) and glycerol, hence its name. It was identified in 1823 by the French chemist Michel-Eugène Chevreul (1786-1889), who in Recherches chimiques sur les corps gras d'origine animale explained the chemical reaction of saponification and the composition of the stearin. He showed that triglycerides can be considered chemical combinations between glycerol and fatty acids (in other words, a triglyceride is a compound whose molecule contains one glycerol residue and three fatty acid residues).

Mechanism
The reaction mechanism breaks down into three stages (plus an acidification of the medium if it is desired to obtain a carboxylic acid and no longer a carboxylate ion).
- First step: nucleophilic addition of HO ion−the ester.
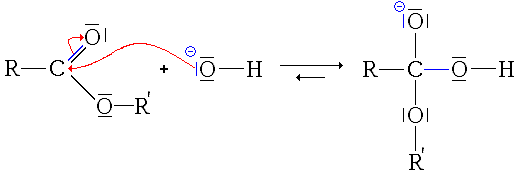
- Second step: elimination of the alcohol group.
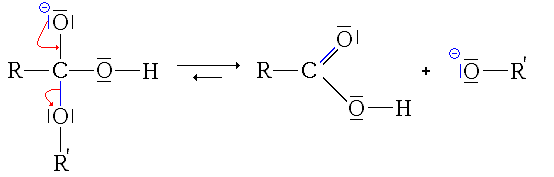
- In this step, the reaction could be completed and have a utility (to hydrolyze a ester, it would be enough to add soda or potassium). This step forms a carboxylic acid, weak acid (pK)a usually between 3 and 4, but relatively stronger in the middle, the other being the water) and a very strong base, the alcohol ion (pKa between 18 and 20). Therefore, there is an acid-base reaction between the strongest acid and the strongest base, therefore, the conversion of carboxylic acid in carboxylate ion.
- Third step: acid-base reaction between carboxylic acid and alcohol ion.
As can be seen, this reaction is the only irreversible and (almost) total mechanism (1014 < K < 1017). Therefore, it shifts the equilibria of the previous reactions (by consuming their products completely), making them also total (or almost).
In the context of the synthesis of a soap, it can be stopped at this last stage. On the other hand, if you want to obtain a carboxylic acid, you have to re-acidify the medium:
Types of lipids by their saponification characteristics
Saponifiable lipids
A saponifiable lipid would be any lipid that is made up of an alcohol attached to one or several (same or different) fatty acids. This union is carried out through an ester bond, which is very difficult to hydrolyze. But it can be easily broken if the lipid is in a basic medium. In this case, alkaline saponification occurs. In cases where a glyceride or neutral fat is used to obtain soap, alcohol called glycerin is obtained as a by-product, which can give greater economic benefits than the main product.
In the example above, a lipid molecule is treated with two potassium hydroxide molecules; Two molecules of potassium palmitate (a soap) and one of glycerin are obtained.
The cleansing action of soap is due to its emulsifying power, that is, its ability to suspend substances in water that normally do not dissolve in pure water. The hydrocarbon chain (hydrophobic part) of salt (soap) has an affinity for non-polar substances, such as fats in food. The carboxylate group (hydrophilic part) of the molecule has an affinity for water.
In the soap solution, carboxylate ions surround the fat droplets: their nonpolar parts sit (dissolve) inward, while carboxylate groups arrange themselves on the outer surface. Thus, reduced to very small volumes, the droplets can associate with the water molecules and the dispersion of the fat is facilitated. These small droplets containing the nonpolar particles surrounded by carboxylate anions are called micelles. It is the presence of these carboxylate anions that causes the surfaces of the micelles to be negatively charged and repel each other, preventing coalescence and maintaining the emulsion, that is, the dispersion in very fine droplets.
Transparency of the soap
The transparency of the soap depends on the fatty acid content. An excess of this makes it opaque and milky in consistency.
When soap is made using a cold process, the soap will come out opaque, even if the measurements for alkalis and oils have been very accurate, as this process rarely produces enough heat to completely neutralize the fatty acids.
The hot process incorporates the heat from the kitchen into the chemical heat produced by saponification. This added heat binds all the fatty acids with the alkali, resulting in a clear, neutral soap.
Saponification is a chemical reaction that produces heat, and the more heat it produces the more complete the saponification.
Contenido relacionado
Plasma (state of matter)
Melamine
Antoine Lavoisier
