Rutherford's experiment
The Rutherford experiments were a series of landmark experiments through which scientists discovered that every atom has a nucleus where its positive charges and most of its mass are concentrated. They deduced this by measuring how a beam of alpha particles spreads out when it hits a thin metal sheet. The experiments were carried out between 1908 and 1924 by Hans Geiger and under the direction of Ernest Rutherford in the laboratories of the University of Manchester.
Summary
The popular theory of nuclear structure was that of JJ Thomson. Thomson was the scientist who discovered the electron that is part of every atom. Thomson believed that the atom was a sphere of positive charge in which the electrons were arranged. Protons and neutrons were unknown at that time.
Thomson's model was not universally accepted. Thomson himself was not able to develop a complete and stable model of his concept. Hantaro Nagaoka, a Japanese scientist, rejected this on the grounds that opposite electrical charges cannot penetrate each other. Instead, he proposed that the electrons orbited the positive charge like Saturn's rings.
The prediction
According to Thomson's model, if an alpha particle (positively charged sub-microscopic particle) collided with an atom, it would pass straight through. At the atomic scale, the concept of "solid matter" is meaningless, so the alpha particle would not bounce off the atom like marbles. It would only be affected by the electric fields of the atom, and in Thomson's model the electric fields were too weak to affect a passing alpha particle to any significant degree. Both negative and positive charges within the Thomson atom extend over the entire volume of the atom. According to Coulomb's Law, the less concentrated a sphere of electric charge is, the weaker its electric field will be at its surface.
As a worked example, consider an alpha particle passing tangentially to a Thomson's gold atom, where it will experience the electric field at its strongest and thus experience the maximum deflection θ. Since the electrons are very light compared to the alpha particle, their influence can be neglected and the atom can be seen as a positively charged sphere.
- Qn = load of a gold atom = 79e = 1.266 × 10- 17 C
- Qα = load of an alpha particle = 2e = 3.204 × 10-19 C
- r = radius of a golden atom = 1.44 × 10-10 m
- vα = speed of an alpha particle = 1.53 107 m/s
- mα = mass of an alpha particle = 6,645 × 10-27 kg
- k = Coulomb Constant = 8.998 × 109 N·m2/C2
Using classical physics, the lateral change of the alpha particle at moment Δp can be approximated using the momentum force relationship and the Coulomb force expression.
- Δ Δ p=FΔ Δ t=k⋅ ⋅ Qα α Qnr2⋅ ⋅ 2rvα α {displaystyle Delta p=FDelta t=kcdot {frac {Q_{alpha }Q_{n}}{r^{2}}}}cdot {frac {2r}{v_{alpha }}}}}}}}}
- <math alttext="{displaystyle theta approx {frac {Delta p}{p}}θ θ ≈ ≈ Δ Δ pp.k⋅ ⋅ 2Qα α Qnmα α rvα α 2=8.998⋅ ⋅ 109× × 2× × 3.204⋅ ⋅ 10− − 19× × 1.266⋅ ⋅ 10− − 176.645⋅ ⋅ 10− − 27× × 1.44⋅ ⋅ 10− − 10× × (1.53⋅ ⋅ 107)2{displaystyle theta approx {frac {delta p}{p}}{cdot {frac {2Q_{alpha }Q_{n}}{m_{alpha }{cHFF}{cHFFFF}{cHFF}{cHFFFF}{cHFF}{cHFF}{cHFFFFFFFF}{cHFF}{cHFF}{cHFFFFFF}{cHFF}{cHFFFF}{cHFFFFFFFFFFFFFFFFFF}{cHFF}{cHFF}{cHFF}{cHFFFFFFFFFFFFFFFF}{cHFF}{cHFF}{cHFF}{cHFFFF}{cHFF}{cHFFFFFF}{cHFF}{cHFF}{cHFF}{cHFFFFFFFF}{cH<img alt="{displaystyle theta approx {frac {Delta p}{p}}
- <math alttext="{displaystyle theta θ θ .0.000326rad(orr0.0186 ){displaystyle theta ₡0.000326~mathrm {rad} ~(mathrm {or} ~0.0186^{circ })}<img alt="{displaystyle theta
The above calculation is only an approximation, but it is clear that the deflection will at most be on the order of a small fraction of a degree. If the alpha particle were to pass through gold foil some 400 atoms thick and experience a maximum deflection in the same direction (unlikely), it would still be a small deflection.
The result
At Rutherford's request, Geiger and Marsden conducted a series of experiments in which they directed a beam of alpha particles at a thin piece of gold foil and measured the scattering pattern using a fluorescent screen. They detected alpha particles bouncing off the gold foil in all directions, some back at the source. This should be impossible according to Thomson's model. Obviously, these particles had encountered an electrostatic force much greater than Thomson's model, which in turn implied that the atom's positive charge was concentrated in a much smaller volume than Thomson imagined.
When Geiger and Marsden shot alpha particles into their foils, they found that only a small fraction of the alpha particles were deflected by more than 90°. Most flew right through the foil. This suggested that these tiny spheres of intense positive charge were separated by vast gulfs of empty space. Most of the particles passed through empty space with minimal deflection, and a small fraction hit the nuclei and were strongly deflected.
Rutherford thus rejected Thomson's model, and instead proposed a model in which the atom consisted of mostly empty space, with all its positive charge concentrated in the center of a very small volume, surrounded by a cloud of electrons..
Summary: Most of the alpha rays passed through the sheet without splitting, most of the space of an atom is empty space. There is a tiny, dense region that he called the nucleus, which contains positive charge and almost all the mass of the atom; some rays were deflected because they pass very close to the center with an electrical charge of the same type as alpha rays (positive charge); very few bounced because they collided head-on with centers of positive charge.
Personal schedule
Context:
Ernest Rutherford was a professor of physics at the University of Manchester. He had already received numerous honors for his radiation studies. He had discovered the existence of alpha rays; beta rays and gamma rays, and he had shown that these were the consequence of the disintegration of atoms. In 1906, he was visited by a German physicist named Hans Geiger, and he was so impressed that he asked Geiger to stay and help with his research. Ernest Marsden was a physics undergrad studying under Geiger.
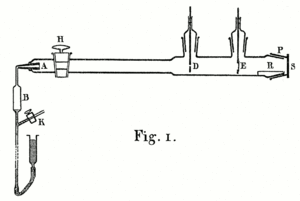
Alpha particles are small positively charged particles that are spontaneously emitted by certain substances such as uranium and radium. Rutherford himself had discovered them in 1899. In 1908 he was trying to accurately measure their charge-mass ratio. To do this, he first needed to know how many alpha particles his radio sample was emitting (after which he would measure their total charge and divide one by the other). Alpha particles are too small to be seen even with a microscope, but Rutherford knew that alpha particles ionize air molecules, and if the air is within an electric field, the ions will produce an electric current. On this principle, Rutherford and Geiger designed a simple counting device consisting of two electrodes in a glass tube. Each alpha particle that passed through the tube created a pulse of electricity that could be counted. It was an early version of the Geiger counter.
The experiments they designed involved bombarding a metal foil with alpha particles to observe how the foil scattered them relative to its thickness and material. They used a fluorescent screen to measure the trajectories of the particles. Each impact of an alpha particle on the screen produced a small flash of light. Geiger worked in a darkened laboratory for hours on end, counting these tiny sparkles under a microscope. Rutherford lacked the stamina for this work, so he left it to his younger colleagues. For the metal foil, they tried a variety of metals, but preferred gold because they could make the foil very thin, since gold Gold is very malleable. As a source of alpha particles, Rutherford's substance of choice was radium, a substance several million times more radioactive than uranium.
The 1908 Experiment
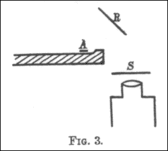
A 1908 paper by Geiger, "On the Scattering of α-Particles by Matter", describes the following experiment. Geiger built a long glass tube almost two meters long. At one end of the tube was a quantity of "radio emanation" (R) that served as a source of alpha particles. The opposite end of the tube was covered with a phosphorescent screen (Z). In the center of the tube was a slit 0.9 mm wide. Alpha particles from R passed through the slit and created a bright patch of light on the screen. A microscope (M) was used to count the scintillations on the screen and measure their propagation. Geiger pumped all the air out of the tube so that the alpha particles were unclogged and left a clean, tight image on the screen that corresponded to the shape of the slit. Geiger then let some air into the tube, and the bright patch became more diffuse. Geiger then pumped out the air and placed gold leaf over the slot at AA. This also caused the light patch on the screen to spread out more. This experiment demonstrated that both air and solid matter could remarkably scatter alpha particles. The apparatus, however, could only observe small angles of deflection. Rutherford wanted to know if the alpha particles were being scattered at even larger angles—perhaps more than 90°.
The 1909 Experiment
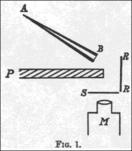
In a 1909 paper, "On a Diffuse Reflection of Alpha Particles", Geiger and Marsden described the experiment by which they showed that alpha particles can be scattered by more than 90°. In their experiment they prepared a small conical glass tube (AB) containing radium, and its opening was sealed with mica. This was your alpha particle emitter. They mounted a lead plate (P), behind which a fluorescent screen (S) was placed. They positioned the radio tube on the other side of the plate in such a way that the alpha particles it emitted could not directly hit the screen. They noticed a few flashes on the screen. It was because some alpha particles avoided the lead plate by bouncing off the air molecules. They then attached a sheet of metal (R) to the side of the lead plate. They noticed more flickering on the screen because the alpha particles were bouncing off the foil. Counting the scintillations, they noticed that metals with higher atomic masses, such as gold, reflected more alpha particles than lighter ones, such as aluminum.
Geiger and Marsden then wanted to estimate the total number of alpha particles that were being reflected. The previous setup was not suitable for this because the tube contained various radioactive substances (radium and its decay products) and therefore the emitted alpha particles had varying ranges and because it was difficult for them to determine at what speed the tube was emitting particles. alpha. This time, they placed a small amount of radium C (bismuth-214) on a lead plate, which bounced off a platinum (R) reflector and onto the screen. They found that only a small fraction of the alpha particles that hit the reflector bounced off the screen (1 in 8,000).
The 1910 Experiment
A 1910 paper by Geiger, "The Scattering of α-Particles by Matter," describes an experiment in which he attempted to measure how the most likely angle through which an alpha particle deviates varies with material by the passing, the thickness of the material, and the velocity of the alpha particles. Geiger constructed an airtight glass tube from which air was pumped. At one end was a bulb (B) containing "radium emanation" (radon-222). By means of mercury, the radon in B was pumped through the tube slit onto a fluorescent zinc sulfide (S) screen. The microscope he used to count the flashes on the screen was set to a vertical millimeter scale with a vernier, which allowed Geiger to precisely measure where the flashes of light appeared on the screen and thus calculate the angles of the light particles. deflection. Alpha particles emitted from A narrowed to a beam through a small circular hole at D. Geiger placed a sheet of metal in the path of the rays at D and E to observe how the flash zone changed. He could also vary the speed of the alpha particles by placing extra sheets of mic or aluminum in A.
From the measurements he took, Geiger came to the following conclusions:
- the most likely deflection angle increases with the thickness of the material
- the most likely deflection angle is proportional to the atomic mass of the substance
- the most likely deflection angle decreases with alpha particle speed
- the probability that a particle deviates by more than 90° is very small
Rutherford mathematically models the dispersion pattern
In 1911, Rutherford published a landmark paper in 1911 titled "The Scattering of Alpha and Beta Particles by Matter and the Structure of the Atom" in which he proposed that the atom contains at its center a volume of electrical charge that is very small and intense (Rutherford treated it as a point charge in his equations). For the purposes of his equations, he assumed that this central charge was positive, but admitted that he could not prove this yet.
Rutherford developed an equation that modeled how the foil should scatter alpha particles if all the positive charge and most of the atomic mass were concentrated at a single point in the center of an atom.
s=Xntcsc4 φ φ 216r2⋅ ⋅ (2QnQα α mv2)2{displaystyle s={frac {Xntcscsc ^{4}{tfrac {phi }{2}}{16r^{2}}}}{cdot ({frac {2Q_{n}Q_{alpha }}{mv^{2}}}{2}}{2}}}}
- s = the number of alpha particles falling over the unitary area with a deflection angle
- r = distance from the point of incidence of alpha rays on the dispersal material
- X = total number of particles falling on the dispersal material
- n = the number of atoms in a unitary volume of the material
- t = thickness of the sheet
- Qn = positive load of the atomic nucleus
- Qα = the positive load of alpha particles
- m = mass of an alpha particle
- v = the speed of the alpha particle
The gold foil
In a 1913 paper, "The Laws of Deflection of α-Particles by Large Angles", Geiger and Marsden describe a series of experiments by which they attempted to experimentally verify the above equation that Rutherford developed. Rutherford's equation predicted that the number of flashes per minute (s) to be observed at a given angle (Φ) should be proportional to:
- csc4≈/2
- thickness of the sheet t
- magnitude of the central load Qn
- 1/(mv2)2
His 1913 paper describes four experiments by which they each demonstrated these four relationships.
To test how the scattering varied with deflection angle (i.e., if s ∝ csc4Φ/2) Geiger and Marsden built an apparatus consisting of a hollow metal cylinder mounted on a turntable. Inside the cylinder were a metal foil (F) and a radon-containing radiation source (R), mounted on a separate column (T) that allowed the cylinder to rotate independently. The column was also a tube through which air was pumped out of the cylinder. A microscope (M) with its objective covered by a fluorescent screen of zinc sulphide (S) penetrated the wall of the cylinder and was aimed at the metal sheet. By rotating the table, the microscope can be moved in a circle around the slide, allowing Geiger to observe and count alpha particles deflected up to 150°. Correcting for experimental error, Geiger and Marsden found that the number of alpha particles that are deflected by an angle Φ is indeed proportional to csc4Φ/2.
Geiger and Marsden then proved how the scattering varied with the thickness of the sheet (i.e. if s ∝ t). They built a disc (S) with six holes drilled into it. The holes were covered with metal sheets of varying thickness, or none for the control. This disc was then sealed in a brass ring (A) between two glass plates (B and C). The disk could be rotated by means of a bar (P) to bring each window in front of the alpha particle source (R). A zinc sulfide (Z) screen was located in the rear glass panel. Geiger and Marsden observed that the number of twinkles that appeared on the screen was actually proportional to the thickness, as long as the thickness was small.
Geiger and Marsden reused the above apparatus to measure how the scattering pattern varied with the square of the nuclear charge (i.e. if s ∝ Qn2 ). Geiger and Marsden assumed that the charge of the nucleus was proportional to the atomic weight of the element, so they tested whether the dispersion was proportional to the atomic weight squared. Geiger and Marsden covered the holes in the disk with sheets of gold, tin, silver, copper, and aluminum. They measured the stopping power of each sheet by equating it to an equivalent thickness of air. They counted the number of flashes per minute that each plate produced on the screen. They divided the number of flashes per minute by the air equivalent. They counted the number of flashes per minute that each plate produced on the screen. They divided the number of flashes per minute by the air equivalent of the respective sheet, then divided again by the square root of the atomic weight (they knew that for sheets of equal stopping power, the number of atoms per unit area is proportional to the square root of the atomic weight). Thus, for each metal, Geiger and Marsden obtained the number of scintillations produced by a fixed number of atoms. For each metal, they then divided this number by the square of the atomic weight, and found that the proportions were more or less equal. Thus they proved that s ∝ Qn2.
Finally, Geiger and Marsden tested how the scattering varied with the velocity of the alpha particles (i.e. if s α 1/v4). Again using the same apparatus, they retarded the alpha particles by placing additional sheets of mica in front of the alpha particle source. They observed that, within the range of experimental error, the number of scintillations was actually proportional to 1/v4.
Rutherford determines that the nucleus is positively charged
In his 1911 paper, Rutherford assumed that the central charge of the atom was positively charged, but acknowledged that he could not say for sure, as a negative or positive charge would have suited his scattering model. other experiments confirmed his hypothesis. In a 1913 paper, Rutherford stated that the "nucleus" was positively charged, based on the result of experiments exploring the scattering of alpha particles in various gases.
In 1917, Rutherford and his assistant William Kay began to explore the passage of alpha particles through gases such as hydrogen and nitrogen. In an experiment where they shot a beam of alpha particles through hydrogen, the alpha particles knocked the hydrogen nuclei forward in the direction of the beam, not backwards. In an experiment shooting alpha particles through nitrogen, he discovered that alpha particles knocked hydrogen nuclei (i.e. protons) out of nitrogen nuclei.
Contenido relacionado
Quantum electrodynamics
Robert Andrews Millikan
Astrophysics