Rhodopsin
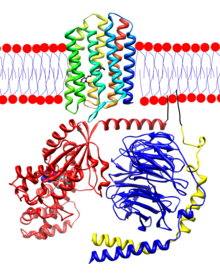
Rhodopsin is a transmembrane protein that, in humans, is found in the rod discs of the retina, specifically in the plasmatic membrane of the rods, in which it is synthesized, forming up to 80% of it.
The rods, together with the cones, form the set of photoreceptor cells of the retina, located on the walls of the retina.
Rhodopsin is the photoluminic receptor that is responsible for absorbing light of wavelengths around 560 nm as part of the image capture process of the eye, and through a series of complex reactions, allows vision, both in normal lighting conditions, such as in spaces with poor lighting.
It consists of a protein part, opsin, and a non-protein part that is a derivative of vitamin A, which is 11-cis-retinal. It is unstable and readily reacts with light energy, discoloring and decomposing on contact with it, and regenerating in the dark.
One of the reactions that happens when it is exposed to light is called phototransduction, and it happens in different ways in invertebrates and vertebrates.
In the case of the former, rhodopsin is associated with the cell membrane, and phototransduction occurs in the cytoplasm, the intracellular aqueous medium.
On the other hand, in vertebrates it crosses the membrane and receives light with the help of an associated G protein that allows the reaction.
The latter is the process through which rhodopsin passes in humans in order to see.
Most non-photosynthetic marine microorganisms capture energy from sunlight through rhodopsin.[citation needed] The protein enables these organisms to use the sun's energy to move, grow and survive in the face of lack of nutrients. Rhodopsin is highly conserved and present in all three major domains (archaea, bacteria, and eukaryotes), suggesting an early appearance and a pivotal role in evolution.
This substance is a combination of the protein scotopsin and the carotenoid pigment retinal (also called retinene). Also, retinal is of a special type called 11-czs-retinal. (Hall & Guyton, 2011)
Composition
The molecular structure is made up of two components: a protein component, the opsin; and one non-protein, 11 cis retinal.
Opsin is a polypeptide chain made up of about 348 amino acids. The opsin is distributed into seven alpha-helical stretches that lie perpendicular to the membrane, linked by structureless protein parts. It is also called visual purple, due to its color and the fact that it was first found in the retina of Franz Boll frogs.
The terminal carboxyl is located in the cytosolic part and the amino in an intradiscal position.
11 cis retinal is synthesized by a complex process through vitamin A, an antioxidant that we obtain with the help of a varied diet rich in fruits and vegetables, and animal products such as liver or whole milk. This is attached to one of the alpha helices in the center of the molecule and placed perpendicularly. This placement causes the photosensitive pigment to emerge, and when light hits the 11-cis-retinal and it transforms, producing reactions that lead to a nerve impulse.
These two components, however, remain together only in conditions of absence of light, and once they capture a photon, an elementary particle responsible for electromagnetic phenomena, it modifies the structure of the protein, separating it.
Synthesis and decomposition
Rhodopsin is formed in the inner part of the retinal rods, that is, in the cell's cytoplasm, specifically in the area of the endoplasmic reticulum, a cellular organelle in charge of synthesizing proteins -such as opsin- and lipids. Once synthesized in it, it passes to the membrane of the Golgi apparatus, and undergoes a transformation.
At the end, the vesicles, organelles with the main function of storing and transporting substances, transport the rhodopsin so that it can fuse with the plasmatic membrane of the retinal photoreceptor cells.
Rhodopsin remains in the rod membrane until photoactivation occurs, and the photopigments capture a photon. This light-sensitive protein is broken down and during this process, the light stimulus received by the photon transforms it into a nerve impulse that generates an electrical signal in the brain that triggers a series of reactions known as the phototransduction process.
Function
The rods, where rhodopsin is found, are cells that are extremely sensitive to light and saturate quickly and easily. This causes them not to distinguish between colors and therefore, in low light conditions our visual spectrum of colors is reduced to almost black and white, and a large scale of greys; but still we can see.
This, however, is not its exclusive function, since some photosynthetic living beings use this pigment to generate energy, through the process of photosynthesis, and to be able to carry out their vital functions, as well as move, feed or interact with the environment that surrounds it.
Contenido relacionado
Symbiosis
Protoplasm
Cerebellum