Radical (chemistry)
In chemistry, a radical (previously free radical) is a chemical species (organic or inorganic), characterized by having one or more unpaired electrons. It is formed in the middle of chemical reactions, from the homolytic rupture of a molecule and, in general, it is extremely unstable and, therefore, with great reactive power and a very short half-life (milliseconds /).
Nomenclature
In the nomenclature of organic compounds, according to the International Union of Pure and Applied Chemistry (IUPAC), the term radical must be used without the adjective free, considered unnecessary and obsolete especially in organic chemistry. In the past, the term radical was used to name a substituent group, and the discovery of its "free" led to the use of the adjective to differentiate them. Currently these substituents are named by their groups, for example alkyl group or methyl group, and the "free radicals" they are named simply radicals.
History
In 1900, Moses Gomberg, professor of chemistry at the University of Michigan, made a series of observations of the reaction of triphenylmethane halides with silver and zinc, in benzene, correctly proposing that it was responsible for the yellow coloration of the highly reactive product in solution was the triphenylmethyl radical.
In 1929, Friedrich Paneth and Wilhelm Hofeditz produced the methyl radical (CH3). Unlike the triphenylmethyl radical, the methyl radical was elusive and could not be isolated, demonstrating that organic free radicals can exist momentarily and suggesting that many other organic chemical reactions may involve radicals.
In 1933, Kharasch and Mayo published their experiments with allyl bromide and hydrogen bromide, in which the presence or absence of oxygen generated different isomers. This was called the "peroxide effect", which was explained by Kharasch in 1936, by an intermediate reaction with the bromine radical Br .
In 1934, Rice and Herzfeld classified the reactions that produced free radicals as initiation if a free radical is formed, propagation if the number of radicals is conserved. free with product formation, of inhibition if the number of free radicals with product disappearance is conserved; and termination if two free radicals disappear when their unpaired electrons combine.
In 1939, Leonor Michaelis proposed that the oxidation of all bivalent organic molecules occurs with the formation of an intermediate free radical. She demonstrated the formation of semiquinones in the oxidation of benzoquinones and naphthoquinones.
In 1946, Michaelis described the sequential univalent reduction of oxygen as a molecular mechanism of four electron transfer steps, with formation of superoxide radical (O2-), hydrogen peroxide (H2O2), and hydroxyl radical (HO·) as the intermediates of the partial reduction of oxygen and with formation of water as the final product of the reduction.
- O2 →2- → H2O2 → HO· → H2O
In 1954, Rebeca Gerschman, while working at the University of Rochester, published the article “Oxygen poisoning and X-irradiation: a mechanism in common” in the journal Science. The "Gerschman theory" postulated that:
- Oxygen-free radicals are the common mechanism of oxygen and radiation toxicities.
- An increase in partial oxygen pressure or a decrease in antioxidant defense also lead to cell and tissue damage.
- Oxygen toxicity is a continuous phenomenon.
In 1969, McCords and Fridovich discovered the enzyme superoxide dismutase isolated from bovine erythrocytes, which catalyzes the dismutation reaction of superoxide into hydrogen peroxide (hydrogen peroxide) and oxygen:
- 2O2-(O)2+ O2·) + 2H+ → H2O2 + O2
The existence of superoxide dismutase implied the immediate recognition of the physiological existence of the superoxide radical, based on the teleology that the enzyme implies the existence of the substrate.
General characteristics
Radicals have independent existence even though they have very short half-lives, so they can be synthesized in the laboratory, they can be formed in the atmosphere by radiation, and they are also formed in living organisms (including the human body) by contact with oxygen and act by altering cell membranes and attacking the genetic material of cells, such as DNA.
Radicals have an open-shell electronic configuration, so they carry at least one unpaired electron that is highly susceptible to creating a bond with another atom or atoms in a molecule. They play important roles in combustion, polymerization, atmospheric chemistry, inside cells, and other chemical processes.
To write chemical equations, radicals are often written by placing a dot (indicating the odd electron) immediately to the right of the atomic symbol or molecular formula as:
- H2 + ♪ → 2 H· (reaction 1)
This is derived from the Lewis notation.
Types of radicals
According to the number of atoms
The radicals can be:
- Monoatomics, like the radical chlorine Cl·The Radical Bromo Br·or radical hydrogen H·which are simply atoms or ions with an odd number of electrons.
- Polyatomics, formed by more than one atom, such as the radical methyl, CH3·
According to the central atom that has the odd electron
Depending on which is the central atom that has the unpaired electron, the radicals can be:
- Carbon-focused Radicals: As a Radical Alchelo (e.g., the Radical Methyl .CH3Or a radical arilo. Within the radicals in C it is appropriate to distinguish, depending on the carbon that the electron is disappeared, between primary radicals (such as the radical methyl CH3·), secondary radicals (such as the radical); and tertiary radicals (such as the tripheylmethyl radical). Tertiary radicals are more stable than secondary ones, and these in turn are more stable than primary ones.
| Primary | Secondary | Ground floor |
|---|---|---|
 |  | |
| Radical etinilo | Radical 2.o derivative 1-bromopropane | Radical trifenylmethyl |
- Nitrogen-focused Radicals: Like the Radical Nitrate ·NO3
- Radicals focused on oxygen: like hydroxyl radical ·OHVery reactive.
- Radicals focused on halogen atom: like the radical chlorine Cl·
- Radicals focused on metal atom: like radical ·SnH3
According to the load
Radicals can be neutral, anionic or cationic, depending on whether they have no charge; or that it is negative or positive.
| Neutral radical | Radical anion | Radical cation |
|---|---|---|
 | 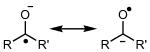 |  |
| Radical metyl (neutro) | Radical anion cetilo (resonance effect) | Formal radical cation |
Radical reactions
They are reactions in which radicals intervene, generally as intermediate states, such as the radical halogenation of alkanes.
General mechanism of radical substitution
Reactions involving free radicals are called radical reactions. They are usually divided into three phases: initiation, propagation, and termination.
The overall substitution reaction shown in equation 1 can be broken down into the following processes:
Initiation reactions These are the reactions that produce an increase in the number of free radicals.
- Equation 2 corresponds to a homopolar rupture caused by thermolysis or photolysis.
- Equation 3 corresponds to a break favored by a radical initiator Init·
Propagation reactions: Reactions between radicals take place. Corresponds to stages 4 and 5.
Termination reactions: Finally, the radicals recombine to form more stable molecules. Corresponds to stages 6 and 7.
Production of radicals in living beings
Radicals are produced in respiration with the presence of oxygen which, although it is essential for the cellular life of our organism, also induces the formation of these reactive molecules, which cause negative health effects throughout life due to to its ability to alter DNA (genes), proteins and lipids or fats (oxidation). In our body there are cells that are continually renewed, such as the cells of the skin, the intestine, and the liver. Over the years, free radicals can produce a genetic alteration in cells that divide continuously, contributing to increase the risk of cancer due to genetic mutations, or they decrease the functionality of cells that do not divide so much, decreasing the number of mitochondria, which is characteristic of aging. Situations that increase the production of free radicals are:
- Environmental pollution.
- The smoking.
- Fat-rich diets.
- Excessive exposure to solar radiation.
- The intake of vegetable oils that were refined, since these contain free radicals when subjected to high temperatures.
- Stress.
Contenido relacionado
Katal
Stable isotope
Actinium
