Polyoxometalate
The polyoxometalates (abbreviated POMs) are inorganic clusters of anionic character and are formed mainly by oxygen and transition metals (M) in a high oxidation state usually, but not necessarily, the highest. The most common metals in polyoxometalates are Mo and W, although others can also be found to a lesser extent such as V, Ti, Zr, etc. Apart from these metals, other elements may be present in the structure, they are called heteroatoms (X) and are usually Al, Si, P or S. POMs can be described as discrete fragments of metal oxides, of well-defined size and shape., formed by the condensation reaction of coordination complexes, generally octahedrons, tetrahedrons, and square-based pyramids. These metallic clusters have a wide range of physical and chemical properties, and can act as building blocks for new materials.
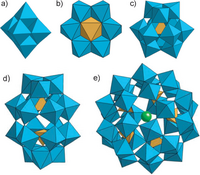
Polyoxometalates range in size from those containing a small number of metal centers, for example the Lindqvist-type anion: (M6O192 -), to those that contain a large number of metals such as the Preyssler type anion: (NaP5W30O110 14-) (see figure). There are also giant polyoxomolybdates that have been extensively studied by Prof. Achim Müller and collaborators.
They are interested in its practical applications, for example:
- redox and acid-base catalysis
- in biomedicine as enzyme inhibitors, such as antiretroviral and antitumoral agent,
- as a protein precipitator agent,
- in some analytical chemistry procedures
- electrochemical
and as model compounds for magnetochemical studies. They have been the subject of numerous theoretical studies with quantum chemistry tools.
Contenido relacionado
Ketone (chemistry)
Diamond
2051