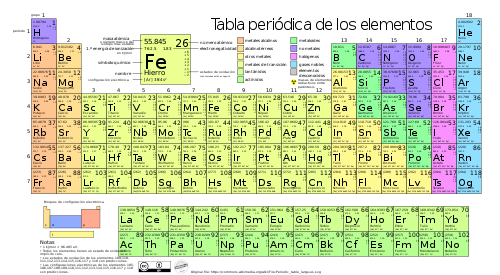
Periodic table of elements
The periodic table of the elements is an arrangement of the chemical elements in table form, ordered by their atomic number (number of protons), by their electron configuration and their chemical properties. This ordering shows periodic trends as items with similar behavior in the same column.
In the words of Theodor Benfey, the periodic table and law "are the heart of chemistry — comparable to the theory of evolution in biology (which succeeded the concept of scala naturae ), and to the principles of thermodynamics in classical physics—».
The rows of the table are called periods and the columns are called groups. Some groups have names, for example group 17 is for the halogens and group 18 is for the noble gases. The table is also divided into four blocks with some similar chemical properties. Because the positions are ordered, the table can be used to derive relationships between the properties of elements, or predict properties of new elements not yet discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior and is widely used in chemistry and other sciences.
Dmitri Mendeleyev published the first widely recognized version of the periodic table in 1869, he developed it to illustrate periodic trends in the properties of the elements then known, by ordering the elements based on their chemical properties, although Julius Lothar Meyer, working separately, carried out an ordering based on the physical properties of atoms. Mendeleyev also predicted some properties of then-unknown elements that he anticipated would occupy the empty places in his table. Most of his predictions were later shown to be correct when the elements in question were discovered.
Mendeleev's periodic table has since been expanded and improved with the discovery or synthesis of new elements and the development of new theoretical models to explain chemical behavior. The current structure was designed by Alfred Werner based on Mendeleev's version. There are also other periodic arrangements according to different properties and according to the use that you want to give it (in didactics, geology, etc.). To celebrate the 150th anniversary of its creation, UNESCO declared 2019 as the International Year of the Table Periodic of the Chemical Elements.
All elements with atomic numbers from 1 (hydrogen) to 118 (oganeson) have been discovered or synthesized; IUPAC confirmed elements 113, 115, 117, and 118 on December 30, 2015, and their official names and symbols were made public on November 28, 2016. The first 94 exist naturally, although some have only been found in small amounts and were synthesized in the laboratory before being found in nature. Elements with atomic numbers 95 to 118 have only been synthesized in laboratories. Numerous synthetic radioisotopes of naturally occurring elements were also produced there. Elements 95 to 100 existed in nature in the past, but do not currently. Research to find new elements of higher atomic numbers by synthesis continues.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Block | s | d | p | |||||||||||||||
| ↓Period | ·The helium belongs to the block s | |||||||||||||||||
| 1 | 1 H | 2 He· | ||||||||||||||||
| 2 | 3 Li | 4 Be | 5 B | 6 C | 7 N | 8 O | 9 F | 10 Ne | ||||||||||
| 3 | 11 Na | 12 Mg | 13 Al | 14 Yeah. | 15 P | 16 S | 17 Cl | 18 Ar | ||||||||||
| 4 | 19 K | 20 Ca | 21 Sc | 22 Ti | 23 V | 24 Cr | 25 Mn | 26 Fe | 27 Co | 28 Ni | 29 Cu | 30 Zn | 31 Ga | 32 Ge | 33 As | 34 Separate | 35 Br | 36 Kr |
| 5 | 37 Rb | 38 Mr. | 39 And | 40 Zr | 41 Nb | 42 Mo | 43 Tc | 44 Ru | 45 Rh | 46 Pd | 47 Ag | 48 Cd | 49 In | 50 Sn | 51 Sb | 52 You | 53 I | 54 Xe |
| 6 | 55 Cs | 56 Ba | 57-71 ♪ | 72 Hf | 73 Ta | 74 W | 75 Re | 76 You | 77 Go | 78 Pt | 79 Au | 80 Hg | 81 Tl | 82 Pb | 83 Bi | 84 Po | 85 At | 86 Rn |
| 7 | 87 Fr | 88 Ra | 89-103 ** | 104 Rf | 105 Db | 106 Sg | 107 Bh | 108 Hs | 109 Mt | 110 Ds | 111 Rg | 112 Cn | 113 Nh | 114 Fl | 115 Mc | 116 Lv | 117 Ts | 118 Og |
| 8 | 119 Uue | |||||||||||||||||
| Block | f | d | |||||||||||||||
| ♪ | Lantana | 57 La | 58 Ce | 59 Pr | 60 Nd | 61 Pm | 62 Sm | 63 Eu | 64 Gd | 65 Tb | 66 Dy | 67 Ho | 68 Er | 69 Tm | 70 Yb | 71 Lu | |
| ** | Arrested | 89 Ac | 90 Th | 91 Pa | 92 U | 93 Np | 94 Pu | 95 Am | 96 Cm | 97 Bk | 98 Cf | 99 That's it. | 100 Fm | 101 Md | 102. No. | 103 Lr | |
| Legend | State of aggregation of matter to 0°C and 1 atm (According to the color of the atomic number) | ||||
| 1 H | . Atomic | Red | Blue | Black | Grey |
| . Chemical symbol | Gaseoso | Liquid | Solid | Unknown | |
| Metals | Metaloids | No metals | |||||||
| Alcalinos | Alcalino-térreos | Lantana | Transmission metals | Other | Other non-metallic | Halogens | Gasesnobles | ||
| Arrested | |||||||||
For a more detailed version of the periodic table with hypertext, see Annex:Recurrent cover.
History
The history of the periodic table is closely related to various aspects of the development of chemistry and physics:
- The discovery of the elements of the periodic table.
- The study of common properties and the classification of the elements.
- The notion of atomic mass (initially called "atomic weight") and, later, already in the centuryXX.d. C., Masonic number.
- The relations between the atomic mass (and, later, the atomic number) and the periodic properties of the elements and the emergence of new elements.
Discovery of the elements
Although some elements such as gold (Au), silver (Ag), copper (Cu), lead (Pb) and mercury (Hg) were already known since antiquity, the first scientific discovery of an element occurred in the 19th century. XVII d. C., when the alchemist Hennig Brand discovered phosphorus (P). In the 18th century d. C. numerous new elements were known, the most important of which were the gases, with the development of pneumatic chemistry: oxygen (O), hydrogen (H) and nitrogen (N). The new concept of element was also consolidated in those years, which led Antoine Lavoisier to write his famous list of simple substances, where 33 elements appeared. At the beginning of the XIX century d. C., the application of the electric battery to the study of chemical phenomena led to the discovery of new elements, such as alkali metals and alkaline-earth metals, especially thanks to the work of Humphry Davy. In 1830, 55 elements were already known. Later, in the middle of the XIX century d. C., with the invention of the spectroscope, new elements were discovered, many of them named by the color of their characteristic spectral lines: cesium (Cs, from the Latin caesĭus, blue), thallium (Tl, from stem, due to its green color), rubidium (Rb, red), etc. During the XX century d. C., research into radioactive processes led to the cascade discovery of a series of heavy elements (almost always artificial substances synthesized in the laboratory, with very short stable life periods), until reaching the figure of 118 elements with names officially accepted by the IUPAC in November 2016.
Notion of element and periodic properties
Logically, a necessary prerequisite for the construction of the periodic table was the discovery of a sufficient number of individual elements, to make it possible to find some pattern in chemical behavior and their properties. During the following two centuries, more knowledge about these properties was acquired, as well as many new elements were discovered.
The word element comes from Greek science, but its modern notion appeared in the 17th centuryd. C., although there is no clear consensus regarding the process that led to its consolidation and widespread use. Some authors cite as a precedent the phrase of Robert Boyle in his famous work The Skeptical Chemist, where he calls elements “certain primitive and simple bodies that are not formed by other bodies, nor from each other, and that are the ingredients of which all perfectly mixed bodies are immediately composed and ultimately resolved. Actually, that phrase appears in the context of Robert Boyle's critique of the four Aristotelian elements.
Throughout the 18th century d. C., the affinity tables included a new way of understanding chemical composition, which is clearly exposed by Lavoisier in his work Elementary Treatise on Chemistry. All of this led to differentiate in the first place which of the substances known up to that moment were chemical elements, what their properties were and how to isolate them.
The discovery of a large number of new elements, as well as the study of their properties, revealed some similarities between them, which increased the interest of chemists in seeking some type of classification.
Atomic Weights
At the beginning of the XIX century d. C., John Dalton (1766-1844) developed a new conception of atomism, which he arrived at thanks to his meteorological studies and atmospheric gases. His main contribution consisted in the formulation of a "chemical atomism" that allowed the integration of the new definition of element made by Antoine Lavoisier (1743-1794) and the ponderal laws of chemistry (defined proportions, multiple proportions, reciprocal proportions).
Dalton used his knowledge of the ratios in which substances of his time reacted and made some assumptions about how atoms in substances combined. He established the mass of a hydrogen atom as the reference unit (although others were suggested in those years) and referred all other values to this unit, so that he was able to construct a system of relative atomic masses. For example, in the case of oxygen, Dalton started from the assumption that water was a binary compound, made up of a hydrogen atom and an oxygen atom. He had no way of checking this point, so he had to accept this possibility as an a priori hypothesis.
Dalton knew that one part of hydrogen combined with seven parts (eight, we would say today) of oxygen to produce water. Therefore, if the combination occurred atom by atom, that is, a hydrogen atom was combined with an oxygen atom, the ratio between the masses of these atoms would have to be 1:7 (or 1:8 would be calculated in the present). The result was the first table of relative atomic masses (or atomic weights, as Dalton called them), which was modified and developed in later years. The aforementioned inaccuracies gave rise to a whole series of controversies and disparities regarding the formulas and atomic weights, which would only begin to be overcome, although not completely, at the Karlsruhe congress in 1860.
First attempts at systematization
In 1789 Antoine Lavoisier published a list of 33 chemical elements, grouping them into gases, metals, non-metals, and earths. Although very practical and still functional in the modern periodic table, it was rejected because there were many differences in both physical and chemical properties.[citation needed]
Chemists spent the next century searching for a more precise classification scheme. One of the first attempts to group elements with similar properties and relate them to atomic weights was made by the German chemist Johann Wolfgang Döbereiner (1780-1849), who in 1817 revealed the remarkable similarity that existed between the properties of certain groups of three elements, with a gradual variation from the first to the last. Later (1827) he pointed out the existence of other groups in which the same relationship was given —chlorine, bromine and iodine; sulfur, selenium and tellurium; lithium, sodium and potassium.
| Lithium | LiCl LiOH | Calcium | CaCl2 CaSO4 | Sulphur | H2S SO2 | ||||||
| Sodium | NaCl NaOH | Stront | SrCl2 SrSO4 | Selenium | H2Separate Seo2 | ||||||
| Potassium | KCl KOH | Bario | BaCl2 BaSO4 | Telurio | H2You I2 |
These groups of three elements were called triads. When classifying them, Döbereiner explained that the average atomic weight of the extreme element weights is similar to that of the element in the middle. This became known as the Law of Triads. For example, for the chloro-bromo-iodine triad, the atomic weights are respectively 36, 80, and 127; the average is 81, which is about 80; the element with an atomic weight of about 80 is bromine, which makes it consistent with the apparent ordering of triads.
The German chemist Leopold Gmelin worked with this system, and by 1843 had identified ten triads, three groups of four, and one group of five. Jean-Baptiste Dumas published work in 1857 describing the relationships between the various groups of metals. Although the various chemists were able to identify the relationships between small groups of elements, they had yet to construct a scheme that would encompass them all.
In 1857 the German chemist August Kekulé observed that carbon is often bonded to four other atoms. Methane, for example, has one carbon atom and four hydrogen atoms. This concept would eventually become known as "valence".
In 1862 de Chancourtois, a French geologist, published a first form of periodic table that he called the "telluric helix" or "screw". He was the first person to notice the periodicity of the elements. By arranging them in a spiral on a cylinder in increasing order of atomic weight, de Chancourtois showed that elements with similar properties seemed to occur at regular intervals. His table also includes some ions and compounds. He also uses geological rather than chemical terms and does not include a diagram; as a result, he received little attention until the work of Dmitri Mendeleev.
In 1864 Julius Lothar Meyer, a German chemist, published a table with 44 elements arranged by valence. It showed that elements with similar properties often shared the same valence. At the same time, William Odling—an English chemist—published an arrangement of 57 elements ordered according to their atomic weights. With some irregularities and gaps, he realized what appeared to be a periodicity of atomic weights among the elements and that this was in accordance with "the groupings they generally received". Odling alludes to the idea of a periodic law, but does not he followed the same. In 1870 he proposed a classification based on the valence of the elements.
Newlands' Law of Octaves
English chemist John Newlands produced a series of papers from 1863 to 1866 noting that when elements are listed in order of increasing atomic weight, similar physical and chemical properties repeat at intervals of eight.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Li 6.9 Na 23,0 K 39,0 | Be 9.0 Mg 24.3 Ca 40,0 | B 10.8 Al 27,0 | C 12,0 Yeah. 28.1 | N 14.0 P 31,0 | O 16,0 S 32.1 | F 19,0 Cl 35.5 |
He compared this periodicity to octaves in music. This so-called "law of octaves" was ridiculed by Newlands's contemporaries and the Chemical Society refused to publish his work, because it ceased to hold from calcium. Newlands was, however, able to produce a table of the elements and used it to predict the existence of missing elements, such as germanium. The Chemical Society only recognized the importance of his discoveries five years after Mendeleyev was credited with them., and was subsequently recognized by the Royal Society, which awarded Newlands its highest decoration, the Davy Medal.
In 1867 Gustavus Hinrichs, a Danish chemist, published a spiral periodic system based on spectra, atomic weights, and other chemical similarities. His work was considered too complicated and therefore he was not accepted.
Mendeleev's periodic table
In 1869, Russian chemistry professor Dmitri Ivanovich Mendeleyev published his first Periodic Table in Germany. A year later Julius Lothar Meyer published an expanded version of the table that he had created in 1864, based on the periodicity of atomic volumes as a function of the atomic mass of the elements.
By this date, 63 elements of the 92 that exist naturally between Hydrogen and Uranium were already known. Both chemists arranged the elements in increasing order of their atomic masses, grouped them into rows or periods of different lengths, and placed in the same group elements that had similar chemical properties, such as valence. They built their tables by listing the elements in rows or columns based on their atomic weight and starting a new row or column when the characteristics of the elements began to repeat themselves.
The recognition and acceptance given to Mendeleev's table came from two decisions he made. The first was to leave gaps when it appeared that the corresponding element had not yet been discovered. He was not the first to do so, but he was renowned for using trends in his periodic table to predict the properties of those missing elements. He even predicted the properties of some of them: gallium (Ga), which he called eka-aluminum because it is located below aluminium; germanium (Ge), which he called eka-silicon; scandium (Sc); and technetium (Tc), which, chemically isolated from synchrotron debris in 1937, became the first predominantly artificially produced element.
The second decision was to ignore the order suggested by the atomic weights and change adjacent elements, such as tellurium and iodine, to better classify them into chemical families. In 1913, Henry Moseley determined the experimental values of the nuclear charge or atomic number of each element, and showed that Mendeleev's order effectively corresponds to that obtained by increasing the atomic number.
The significance of these numbers in the organization of the periodic table was not appreciated until the existence and properties of protons and neutrons were understood. Mendeleev's periodic tables use atomic weight instead of atomic number to organize the elements, information precisely determinable at that time. Atomic weight worked quite well for most cases, allowing us to predict the properties of missing elements with greater precision than any method known at the time. Moseley predicted that the only missing elements between aluminum (Z = 13) and gold (Z = 79) were Z = 43, 61, 72, and 75, which were discovered later. The atomic number sequence is still used today even though new elements have been discovered and synthesized.
Mendeleev's second periodic table and later developments
In 1871, Mendeleev published his periodic table in a new form, with groups of similar elements arranged in columns instead of rows, numbered I to VIII in correlation with the oxidation state of the element. He also made detailed predictions of the properties of elements that he had already pointed out were missing, but should exist.These gaps were later filled when chemists discovered additional natural elements.
In his new table, he states the ordering criteria of the columns are based on the hydrides and oxides that these elements can form and therefore, implicitly, the valences of those elements. It was still giving contradictory results (Silver and Gold appear duplicated, and there is no separation between beryllium and magnesium with boron and aluminum), but it meant a great advance. This table was completed with one more group, made up of the noble gases discovered during Mendeleev's lifetime, but which, due to their characteristics, had no place in the table, for which reason he had to wait almost thirty years, until 1904, with the group or zero valence, leaving the most complete table.
It is often claimed that the last natural element to be discovered was francium—designated by Mendeleev as eka-cesium—in 1939. However, plutonium, produced synthetically in 1940, was identified in trace amounts as an element primordial of natural origin in 1971.
The layout of the standard periodic table is attributable to Horace Groves Deming, an American chemist who in 1923 published an 18-column periodic table. In 1928 Merck and Company prepared a booklet with this table, which was widely circulated in American schools. By the 1930s it was appearing in chemistry textbooks and encyclopedias. It was also distributed for many years by the Sargent-Welch Scientific Company.
Quantum mechanics and progressive expansion of the table
Mendeleev's periodic table had certain irregularities and problems. In subsequent decades he had to integrate the discoveries of noble gases, "rare earths" and radioactive elements. Another additional problem were the irregularities that existed to combine the ordering criterion by increasing atomic weight and the grouping by families with common chemical properties. Examples of this difficulty are found in tellurium-iodine, argon-potassium and cobalt-nickel pairs, in which it is necessary to alter the criterion of increasing atomic weights in favor of grouping into families with similar chemical properties.
For some time, this question could not be satisfactorily resolved until Henry Moseley (1867-1919) made a study of X-ray spectra in 1913. Moseley found that by plotting the square root of the radiation frequency as a function of a straight line was obtained from the order number in the periodic system, which allowed us to think that this order was not accidental, but a reflection of some property of the atomic structure. Today we know that this property is the atomic number (Z) or number of positive charges in the nucleus.
The currently accepted explanation of the periodic law arose after the theoretical developments produced in the first third of the 20th century, when the theory of quantum mechanics was built. Thanks to these investigations and subsequent developments, it is accepted that the arrangement of the elements in the periodic system is related to the electronic structure of the atoms of the various elements, from which their different chemical properties can be predicted.
In 1945 Glenn Seaborg, an American scientist, suggested that the actinides, like the lanthanides, were filling an f subshell instead of a fourth row in the d block, as previously thought. Seaborg's colleagues advised him not to publish such a radical theory, since it would most likely ruin his career. Since he considered that he did not then have a career that could fall into disrepute, he published it anyway. Later it was found that he was correct and in 1951 he won the Nobel Prize in Chemistry for his work on the synthesis of actinides.
In 1952, the Costa Rican scientist Gil Chaverri presented a new version based on the electronic structure of the elements, which allows the lanthanide and actinide series to be located in a logical sequence according to their atomic number.
Although small amounts of some transuranium elements occur naturally, all of them were first discovered in laboratories, the first of which was neptunium, synthesized in 1939. The production of these elements has significantly expanded the table periodic. Because many are highly unstable and decay rapidly, they are difficult to detect and characterize when they occur. There have been controversies regarding the acceptance of discovery claims and rights to some items, requiring an independent review to determine which party has priority, and therefore naming rights. Flerovium (element 114) and livermorium (element 116) were named on May 31, 2012. 117).
On December 30, 2015, the IUPAC officially recognized elements 113, 115, 117, and 118, completing the seventh row of the periodic table. On November 28, 2016, the official names and symbols of the elements were announced. last four new elements approved to date by IUPAC (Nh, nihonium; Mc, muscovium; Ts, teneso; and Og, oganeson), which replace the temporary designations.
Structure and organization of the periodic table
The current periodic table is a system where the elements known to date are classified. They are placed from left to right and from top to bottom in increasing order of their atomic numbers. The elements are arranged in seven horizontal rows called periods, and in 18 vertical columns called groups or families.
Moving down and to the left increases the atomic radius and ionic radius.
Moving up and to the right increases ionization energy, electron affinity, and electronegativity.
Groups
The vertical columns of the table are known as groups or families. There are 18 groups in the standard periodic table. Under an international naming convention, the groups are numbered from 1 to 18 from the leftmost column—the alkali metals—to the rightmost column—the noble gases.
Previously, Roman numerals were used according to the last digit of today's naming convention — for example, elements in group 4 were in the IVB and those in group 14 were in the VAT. In the United States, the Roman numerals were followed by a letter "A" if the group was in the s or p block, or a "B" if it was in the d block. In Europe, letters were used in a similar way, except that "A" was used if it was a group preceding 10, and "B" for 10 or after. Furthermore, groups 8, 9 and 10 used to be treated as a single triple group, collectively known in both notations as group VIII. In 1988 the new IUPAC nomenclature system was put into use and the previous group names were discarded.
Some of these groups have trivial (non-systematic) names, as shown in the table below, although they are not always used. Groups 3 through 10 do not have common names and are referred to simply by their group numbers or the name of their first member—for example, "the scandium group" for 3—since they have fewer similarities. and/or vertical trends.
The modern explanation of the arrangement in the periodic table is that the elements of a group have similar electronic configurations and the same valence, understood as the number of electrons in the last shell. Since chemical properties are highly dependent on the interactions of electrons that are located at the outermost levels, elements in the same group have similar chemical properties and show a clear trend in their properties with increasing atomic number.
For example, elements in group 1 have an electron configuration ns1 and a valence of 1—one outer electron—and they all tend to lose that electron at the same time. bond as positive +1 ions. The elements in the last group on the right are the noble gases, which have their last energy level filled — the octet rule — and are therefore exceptionally unreactive and are also called "inert gases."
Elements in the same group tend to show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, the valence electrons are further from the nucleus. From the top, each successive element has a lower ionization energy, since it is easier to remove an electron in atoms that are less tightly bound. Similarly, a group has a decrease in electronegativity from top to bottom due to an increasing distance between the valence electrons and the nucleus.
There are exceptions to these trends, such as what occurs in group 11, where electronegativity increases further down the group. Also, in some parts of the periodic table such as the d and f blocks, horizontal similarities can be as or more pronounced than the verticals.
Periods
The horizontal rows of the periodic table are called periods. The number of energy levels of an atom determines the period to which it belongs. Each level is divided into different sublevels, which fill up as their atomic number increases in this order:
|
Following that rule, each element is placed according to its electronic configuration and forms the periodic table.
Elements in the same period show similar trends in atomic radius, ionization energy, electron affinity, and electronegativity. In a period the atomic radius normally decreases as we move to the right because each successive element added protons and electrons, which causes the latter to be pulled closer to the nucleus. This decrease in atomic radius also causes the energy of Ionization and electronegativity increase from left to right over a period, due to the attraction of the nucleus on electrons. Electron affinity also shows a slight trend over a period. Metals—on the left—generally have a lower affinity than nonmetals—on the right of the period—except for the noble gases.
The periodic table consists of 7 periods:
|
Blocks
The periodic table can also be divided into blocks according to the sequence in which the electron shells of the elements are filled. Each block is named after the orbital in which the last electron theoretically resides: s, p, d, and f. The s block comprises the first two groups (alkali and alkaline earth metals), as well as hydrogen and helium. The p block comprises the last six groups —which are groups 13 to 18 in IUPAC (3A to 8A in America)— and contains, among other elements, all the metalloids. Block d comprises groups 3 to 12—or 3B to 2B in American group numbering—and contains all the transition metals. The f block, often placed below the rest of the periodic table, has no group numbers and is composed of lanthanides and actinides. There may be more elements that would fill other orbitals, but they have not been synthesized or discovered; in this case, continue with the alphabetical order to name them. This is how block g arises, which is a hypothetical block.
Metals, metalloids and non-metals
Based on the physical and chemical properties they share, the elements can be classified into three broad categories: metals, metalloids, and nonmetals. Metals are generally shiny, highly conductive solids that form alloys with one another, and salt-like ionic compounds with nonmetallic compounds—provided they are not the noble gases. Most nonmetals are colorless or colored gases; They can form covalent bonds with other non-metals. Between metals and non-metals are the metalloids, which have intermediate or mixed properties.
Metals and non-metals can be classified into subcategories that show a gradation from metallic to non-metallic properties, from left to right, in the rows: alkali metals -highly reactive-, alkaline earth metals -less reactive-, lanthanides and actinides, transition metals and post-transition metals. The nonmetals are simply subdivided into polyatomic nonmetals, which, being closer to metalloids, show some incipient metallic character, diatomic nonmetals, which are essentially nonmetals, and the noble gases, which are monatomic nonmetals and nearly so. completely inert. Subgroups within the transition metals are also occasionally noted, such as refractory metals and noble metals.
Placing items into categories and subcategories based on shared properties is imperfect. There is a spectrum of properties within each category and it is not difficult to find overlaps at the boundaries, as is the case with most classification systems. Beryllium, for example, is classified as an alkaline earth metal, although its chemical composition amphoteric and its tendency to form covalent compounds are two attributes of a chemically weak or later transition metal. Radon is classified as a nonmetal and a noble gas, although it has some cationic chemistry more characteristic of a metal. It is also possible to classify based on the division of the elements into categories of events, mineralogical or crystalline structures. The categorization of elements in this way dates back to at least 1869, when Hinrichs wrote that simple boundary lines can be drawn to show elements that have similar properties, such as metals and nonmetals, or the gaseous elements.
Other ways of representing the periodic table
Variants of the composition of group 3
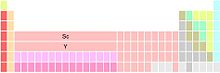
There are three main variants of the periodic table, each different in terms of the constitution of group 3. Scandium and yttrium are shown uniformly, as they are the first two members of this group; the differences depend on the identity of the remaining members.
Group 3 consists of Sc, Y, and La, Ac. Lanthanum (La) and actinium (Ac) occupy the two positions below yttrium (Y). This variant is the most common. It emphasizes the similarities of the periodic trends by moving down groups 1, 2, and 3, at the expense of discontinuities in the periodic trends between groups 3 and 4 and the fragmentation of the lanthanides and actinides.
Group 3 consists of Sc, Y, and Lu, Lr. Lutetium (Lu) and lawrencium (Lr) occupy the two positions below yttrium. This variant preserves a 14-column wide f-block while defragmenting the lanthanides and actinides. It emphasizes the similarities of periodic trends between group 3 and the following groups at the expense of discontinuities in the periodic trends between groups 2 and 3.
Group 3 consists of Sc, Y, and 15 lanthanides and 15 actinides. The two positions below yttrium contain the lanthanides and actinides (possibly because of footnotes). This variant emphasizes the similarities in the chemistry of the 15 lanthanide (La-Lu) elements, at the expense of ambiguity as to which elements occupy the two positions below group 3 yttrium, and apparently of a broad f-block of 15 columns—there can only be 14 elements in any row of the f block—
The three variants stem from historical difficulties in placing the lanthanides in the periodic table, and arguments as to where the f-block elements begin and end. Such arguments have been claimed to be proof that "it is a mistake to break the [periodic] system into strongly bounded blocks". Similarly, some versions of the two-marker table have been criticized for implying that all 15 lanthanides occupy the single box or place below of yttrium, in violation of the basic principle of "one place, one element".
Periodic tables with different structure
The modern periodic table is sometimes expanded to its long or 32-column form by restoring the f-block elements to their natural position between the s- and d-blocks. Unlike the 18-column form, this arrangement results in "the unbroken increase in the sequence of atomic numbers". It also makes it easier to see the relationship of the f-block to the other blocks in the periodic table. Jensen advocates a 32-column table form on the grounds that the lanthanides and actinides are relegated in students' minds as opaque and unimportant items that can be quarantined and ignored. Despite these advantages, editors they generally avoid the 32-column formulation because their rectangular relationship does not adequately accommodate the aspect ratio of a book page.
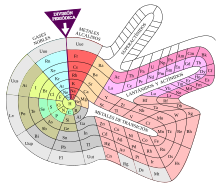
Scientists discuss the efficiency of each periodic table model. Many question even that the two-dimensional distribution is the best. They argue that it is based on convention and convenience, mainly due to the need to fit them to the page of a book and other presentations on the plan. Mendeleev himself was not satisfied and considered the spiral distribution, without luck. Some arguments in favor of new models consist of, for example, the location of the lanthanide and actinide groups outside the body of the table, and even that helium should be located in group 2 of the alkaline earths, since it shares with they have two electrons in their outer shell. For this reason, over the years other periodic tables have been developed in a different order, such as triangle, pyramid, step tables, tower and spiral. The galaxy corresponds to this last type chemistry, Theodor Benfey's spiral and Melinda E Green's spiral-fractal form. It is estimated that more than 700 versions of the periodic table have been published.
According to Phillip Stewart, if Mendeleev had further developed the spiral model, he would have been able to predict the properties of the halogens. Using this idea, Stewart himself created a spiral periodic table that he called a "Chemical Galaxy," accommodating the increasing length of periods in the arms of a spiral galaxy.
In the words of Theodor Benfey, the table and the periodic law
are the heart of chemistry—comparable to what the theory of evolution in biology (which happened to the concept of scala naturae) and the thermodynamic principles in classical physics. However, the standard periodic table as shown in the classrooms and used in textbooks always seemed completely unsatisfactory. With its mammoth lagoons in the first and second periods and the non-united collections of lanthanides and activated floating below the table, the last impression a student would have would be the sense of the periodicity of an element.Theodor Benfey
Their concern, then, was strictly pedagogical. For this reason, he designed an oval periodic table similar to a football field that showed no jumps or floating elements. He arranged the elements in a continuous spiral, with hydrogen in the center and the transition metals, lanthanides, and actinides occupying the tops. peninsulas. However, he was not satisfied with the result, as he did not have enough space for the lanthanides. So in a later redesign he created a protrusion to make room for them and published it in 1964 in the journal of which he was editor-in-chief, Chemistry (chemistry) , of the American Chemical Society. The table was modified to allow for the possibility of accommodating new transuranium elements that had not yet been detected, the existence of which had been suggested by Glenn Seaborg, as well as other minor changes. The Benfey spiral was published in calendars, textbooks, and used by the chemical industry, for which it became popular.
The fractal table is based on the continuity of the characteristics of the element at the end of one row with the one at the beginning of the next, which suggests that the distribution could be better represented with a cylinder instead of partitioning the table in columns. Also, in some cases there were many differences between some elements with low atomic numbers. On the other hand, the table incorporates the family of actinides and lanthanides into the overall layout, placing them where they should be by atomic number, instead of keeping them separated into two floating groups at the end as in the standard table. The result is that the families, instead of following columns, follow radial arcs. This table shows the periodicity by introducing forks at the beginning of periods of length 8, 18 and 32.
Most periodic tables are two-dimensional; however, three-dimensional tables have been known since at least 1862 (pre-dating Mendeleev's two-dimensional table of 1869). More recent examples include Courtines' Periodic Classification (1925), Wrigley's Sheet System (1949), Giguère's periodic helix (1965) and Dufour's periodic tree (1996). Stowe's Periodic Table (1989) has been described as having four dimensions—three spatial and one color.
The various forms of periodic tables can be considered as a continuum in physical chemistry. Towards the end of the chemical continuum one can find, for example, the Rayner-Canham Inorganic Periodic Table (2002), which makes Emphasis on unusual trends, patterns, relationships, and chemical properties. Near the end of the physical continuum is Janet's (1928) extended left-echelon periodic table. It has a structure that shows a closer relationship to the order of electron filling per shell and, by association, quantum mechanics. Somewhere in the middle of the continuum lies the standard periodic table; it is considered to express the best empirical trends in physical state, electrical and thermal conductivity, oxidation numbers, and other properties easily inferred from traditional chemical laboratory techniques.
Additional data and controversies
Elements with no known chemical properties
Elements 108 (hasium), 112 (copernicium), and 114 (flerovium) have no known chemical properties. Other superheavy elements may behave differently than is predicted by extrapolation, due to relativistic effects; for example, flerovium was predicted to possibly exhibit some noble gas-like properties, although it is currently (2016) placed in the carbon group. Later experiments, however, suggest that it behaves chemically like lead, as expected. from its position on the periodic table.
Other extensions to the periodic table
It is not clear if the newly found elements will continue the pattern of the standard periodic table as part of period 8 or will require further adjustments or adaptations. Seaborg expects this period to follow the previously established pattern exactly, such that it would include an s block for elements 119 and 120, a new g block for the next 18 elements, and an additional 30 elements that would continue the current f, d, and p blocks. Physicists such as Pekka Pyykkö have theorized that these additional elements would not follow Madelung's rule, which predicts how electron shells fill up, thus affecting the appearance of the standard periodic table.
Element with the highest possible atomic number
The number of possible elements is not known. In 1911 Elliot Adams, based on the arrangement of the elements in each row of the horizontal periodic table, predicted that elements with atomic weights greater than 256 would not exist—which would be between elements 99 and 100 in today's terms. —.The highest recent estimate is that the periodic table may end shortly after the island of stability, which depending on whether or not it is considered a relativistic model will center around Z = 120 and N = 172 or Z = 124-126 and N = 184, since the span of the periodic table is restricted by proton and neutron drip lines. Other predictions of the end of the periodic table include John Emsley's element 128, element 137 of Richard Feynman, and Albert Khazan's element 155.
- Bohr model
The non-relativistic Bohr model exhibits difficulty for atoms with atomic number greater than 137, since these would require the 1s electrons to travel faster than c, the speed of light, making it inaccurate and not can be applied to these elements.
- Relativistic equation of Dirac
The relativistic Dirac equation has problems for elements with more than 137 protons. For them, the Dirac ground state wavefunction is oscillatory, and there is no difference between the positive and negative energy spectra, as in Klein's paradox. If more precise calculations are made, taking into account size effects finite core, the binding energy is found to exceed the limit for elements with more than 173 protons. For the heavier elements, if the innermost (1s) orbital is not filled, the electric field of the nucleus pulls an electron out of the void, resulting in the spontaneous emission of a positron; however, this does not happen if the innermost orbital is filled, so element 173 is not necessarily the end of the periodic table.
Hydrogen and helium placement
Only following the electron configurations, hydrogen (electronic configuration 1s1) and helium (1s2) are placed in groups 1 and 2, above lithium ([He]2s1) and beryllium ([He]2s2). However, this placement is rarely used outside of the context of electron configurations: when the noble gases—then called "inert gases"—were first discovered around 1900, they were identified as "group 0," reflecting that they were not known to have any chemical reactivity at the time, and helium was placed at the top of that group, because he shared this extreme situation. Although the group changed its formal number, many authors continued to place helium directly above neon, in group 18; one of the examples of such placement is the current IUPAC table. The chemical properties of hydrogen are not very close to those of the alkali metals, which occupy group 1, and for this reason hydrogen is sometimes placed elsewhere: one of the most common alternatives is in group 17. One of the The reasons for this is the strictly univalent, predominantly nonmetallic chemistry of hydrogen, that of fluorine—the element placed at the top of group 17—is strictly univalent and nonmetallic. Sometimes, to show how hydrogen has properties corresponding to both alkali metals and halogens, it can appear in two columns at the same time. It can also appear above carbon in group 14: so placed, it fits well to increasing trends in ionization potential values and electron affinity values, and does not stray too far from the electronegativity trend. Finally, hydrogen is sometimes placed separately from any group because its properties in In general, they differ from those of any group: unlike hydrogen, the other elements of group 1 show extremely metallic behavior; Group 17 elements commonly form salts—hence the word "halogen"; elements in any other group show multivalent chemistry. The other period 1 element, helium, is sometimes placed separately from either group as well. The property that distinguishes helium from the rest of the noble gases—despite its extraordinary inertness being very close to neon and argon — is that, in its closed electron shell, helium has only two electrons in the outermost orbital, while the rest of the noble gases have eight.
Groups included in the transition metals
According to IUPAC a transition metal is "an element whose atom has an incomplete d subshell or which can give rise to cations". According to this definition, all elements in groups 3 to 11 are transition metals and group 12, which includes zinc, cadmium and mercury, is excluded.
Some chemists consider "d-block elements" and "transition metals" to be interchangeable categories, thus including group 12 as a special case of transition metal in which d-electrons are not normally involved in bonding. Chemical bond. The discovery that mercury can use its d-electrons in the formation of mercury(IV) fluoride (HgF4) led some scientists to suggest that mercury can be considered a transition metal. Others, like Jensen, argue that the formation of a compound like HgF4 can only occur under highly abnormal conditions. As such, mercury cannot be considered a transition metal by any reasonable interpretation in the normal sense of the term.
In other cases, there are those who do not include group 3, arguing that they do not form ions with a partially occupied d shell and therefore do not present the characteristic properties of transition metal chemistry.
Elements in group 3 of period 6 and 7
Although scandium and yttrium are always the first two group 3 elements, the identity of the next two elements is not resolved. They are either lanthanum and actinium, or lutetium and lawrencium. There are physical and chemical arguments to support the latter provision, but not all authors are convinced.
Lanthanum and actinium are traditionally represented as the remaining members of group 3. It has been suggested that this design originated in the 1940s, with the advent of periodic tables that depend on the electron configurations of the elements and the notion of differentiation of electrons.
The configurations for cesium, barium, and lanthanum are [Xe]6s1, [Xe]6s2, and [Xe]5d1 6s2. Therefore lanthanum has a differentiating 5d electron and this establishes it "in group 3 as the first member of the d-block for period 6".
In group 3 you see a consistent set of electron configurations: scandium [Ar]3d14s2, yttrium [Kr]4d15s2 and lanthanum. Still in period 6, ytterbium was assigned an electron configuration of [Xe]4f135d16s2 and [Xe]4f 145d16s2 for lutetium, resulting “in a differentiating 4f electron for lutetium and firmly establishing it as the last member from block f for period 6." Matthias describes the placement of lanthanum under yttrium as "an error in the periodic system—unfortunately propagated mostly by the Welch company [Sargent-Welch]... and... everyone copied it." Lavelle refuted this by providing a number of well-known reference books that presented periodic tables in such an arrangement.
Early techniques to chemically separate scandium, yttrium, and lutetium were based on the fact that these elements occurred together in the so-called "yttrium group", while La and Ac occurred together in the "cerium group". Consequently, in the 1920s and 1930s some chemists placed lutetium in group 3 instead of lanthanum.
Subsequent spectroscopic work found that the electron configuration of ytterbium was indeed [Xe]4f146s2. This meant that ytterbium and lutetium had 14 f electrons, "resulting in a differentiating d electron instead of f" for the latter, making it an "equally valid candidate" for the next periodic table position in group 3 below of yttrium. Several physicists in the 1950s and 1960s opted for lutetium, in light of a comparison of several of its physical properties with those of lanthanum. This arrangement, in which lanthanum is the first f-block member, is questioned by some authors, since this element lacks f electrons. However, it has been argued that this is not a valid concern since there are other anomalies in the periodic table, such as thorium, which has no f electrons, but is part of that block. As for lawrencium, its configuration electron was confirmed in 2015 as [Rn]5f147s27p1, representing another periodic table anomaly, regardless of whether is placed in the d or f block, as the potentially applicable p-block position has been reserved for nihonium predicted to have an electron configuration of [Rn]5f146d107s27p1.
Optimal Form
The many different shapes of the periodic table have led to questions about whether there is an optimal or ultimate shape. The answer to this question is believed to depend on whether chemical periodicity has an underlying truth, or is instead the product of subjective human interpretation, dependent on circumstances, beliefs, and the predilections of human observers. An objective basis for chemical periodicity could be established by determining the location of hydrogen and helium, and the composition of group 3. In the absence of objective truth, the different forms of the periodic table can be considered variations of chemical periodicity, each one of which explores and emphasizes different aspects, properties, perspectives, and relationships of and between elements. The ubiquity of the standard periodic table is thought to be a consequence of its design, which has a balance of features in terms of ease of construction and size, and its description of atomic order and periodic trends.
Elements
State of the elements in normal conditions of pressure and temperature (0 °C and 1 atm).
Gas
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Hydrogen | H | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Nitrogen | N | 15 | 2 | 7 | 14 | 7 | 7 | 7 |
| Oxygen | O | 16 | 2 | 8 | 16 | 8 | 8 | 8 |
| Fluor | F | 17 | 2 | 9 | 19 | 9 | 10 | 9 |
| Clothes | Cl | 17 | 3 | 17 | 35 | 17 | 19 | 17 |
| Helio | He | 18 | 1 | 2 | 4 | 2 | 2 | 2 |
| Neon | Ne | 18 | 2 | 10 | 20 | 10 | 10 | 10 |
| Argon | Ar | 18 | 3 | 18 | 40 | 18 | 22 | 18 |
| Krypton | Kr | 18 | 4 | 36 | 84 | 36 | 48 | 36 |
| Xenón | Xe | 18 | 5 | 54 | 131 | 54 | 77 | 54 |
| Radom | Rn | 18 | 6 | 86 | 222 | 86 | 136 | 86 |
Liquids
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Cesio | Cs | 1 | 6 | 55 | 133 | 55 | 78 | 55 |
| Francio | Fr | 1 | 7 | 87 | 223 | 87 | 136 | 87 |
| Mercury | Hg | 12 | 6 | 80 | 201 | 80 | 121 | 80 |
| Galio | Ga | 13 | 4 | 31 | 70 | 31 | 39 | 31 |
| Bro. | Br | 17 | 4 | 35 | 80 | 35 | 45 | 35 |
Transition Elements
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Rutherfordio | Rf | 4 | 7 | 104 | 261 | 104 | 157 | 104 |
| Dubnio | Db | 5 | 7 | 105 | 262 | 105 | 157 | 105 |
| Seaborgio | Sg | 6 | 7 | 106 | 263 | 106 | 157 | 106 |
| Tecnecio | Tc | 7 | 5 | 43 | 99 | 43 | 56 | 43 |
| Bohrio | Bh | 7 | 7 | 107 | 262 | 107 | 155 | 107 |
| Hassio | Hs | 8 | 7 | 108 | 265 | 108 | 157 | 108 |
| Meitnerio | Mt | 9 | 7 | 109 | 266 | 109 | 157 | 109 |
| Darmstadtio | Ds | 10 | 7 | 110 | 271 | 110 | 161 | 110 |
| Roentgenio | Rg | 11 | 7 | 111 | 272 | 111 | 161 | 111 |
| Operating | Cn | 12 | 7 | 112 | 272 | 112 | 160 | 112 |
| Nihonio | Nh | 13 | 7 | 113 | 283 | 113 | 170 | 113 |
| Fleece | Fl | 14 | 7 | 114 | 285 | 114 | 171 | 114 |
| Moscovio | Mc | 15 | 7 | 115 | 288 | 115 | 173 | 115 |
| Livermorio | Lv | 16 | 7 | 116 | 289 | 116 | 173 | 116 |
| I got it. | Ts | 17 | 7 | 117 | 291 | 117 | 174 | 117 |
| Oganeson | Og | 18 | 7 | 118 | 293 | 118 | 175 | 118 |
Lanthanide and actinide elements
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Prometio | Pm | Lanthanido | (-) | 61 | 147 | 61 | 86 | 61 |
| Neptunio | Np | Arrested | (-) | 93 | 237 | 93 | 144 | 93 |
| Plutonio | Pu | Arrested | (-) | 94 | 244 | 94 | 150 | 94 |
| Americio | Am | Arrested | (-) | 95 | 243 | 95 | 148 | 95 |
| Curie | Cm | Arrested | (-) | 96 | 247 | 96 | 151 | 96 |
| Berkelio | Bk | Arrested | (-) | 97 | 247 | 97 | 150 | 97 |
| Californio | Cf | Arrested | (-) | 98 | 251 | 98 | 153 | 98 |
| Einstenio | That's it. | Arrested | (-) | 99 | 252 | 99 | 153 | 99 |
| Fermio | Fm | Arrested | (-) | 100 | 257 | 100 | 157 | 100 |
| Mendelevio | Md | Arrested | (-) | 101 | 258 | 101 | 157 | 101 |
| Nobel Prize | No. | Arrested | (-) | 102. | 259 | 102. | 157 | 102. |
| Laurencio | Lr | Arrested | (-) | 103 | 262 | 103 | 159 | 103 |
Alkali and alkaline earth solids
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Lithium | Li | Alcalino | 2 | 3 | 7 | 3 | 4 | 3 |
| Sodium | Na | Alcalino | 3 | 11 | 23 | 11 | 12 | 11 |
| Potassium | K | Alcalino | 4 | 19 | 39 | 19 | 20 | 19 |
| Blonde | Rb | Alcalino | 5 | 37 | 86 | 37 | 49 | 37 |
| Berilio | Be | Alcalinotérreo | 2 | 4 | 9 | 4 | 5 | 4 |
| Magnesium | Mg | Alcalinotérreo | 3 | 12 | 24 | 12 | 12 | 12 |
| Calcium | Ca | Alcalinotérreo | 4 | 20 | 40 | 20 | 20 | 20 |
| Stront | Mr. | Alcalinotérreo | 5 | 38 | 88 | 38 | 50 | 38 |
| Bario | Ba | Alcalinotérreo | 6 | 56 | 137 | 56 | 81 | 56 |
| Radio | Ra | Alcalinotérreo | 7 | 88 | 226 | 88 | 138 | 88 |
Solids from the scandium, titanium, vanadium and copper families
| Element | Symbol | Group | Period | Atomo | Masa | Protons | Neutrons | Electron |
|---|---|---|---|---|---|---|---|---|
| Scandio | Sc | Scandio | 4 | 21 | 45 | 21 | 24 | 21 |
| Itrio | And | Scandio | 5 | 39 | 89 | 39 | 50 | 39 |
| Lantano | La | Scandio | 6 | 57 | 139 | 57 | 82 | 57 |
| Actinio | Ac | Scandio | 7 | 89 | 227 | 89 | 138 | 89 |
| Titanium | Ti | Titanium | 4 | 22 | 48 | 22 | 26 | 22 |
| Circonio | Zr | Titanium | 5 | 40 | 91 | 40 | 51 | 40 |
| Hafnio | Hf | Titanium | 6 | 72 | 179 | 72 | 105 | 72 |
| Vanadio | V | Vanadio | 4 | 23 | 50 | 23 | 27 | 23 |
| Niobio | Nb | Vanadio | 5 | 41 | 93 | 41 | 52 | 41 |
| Tantalio | Ta | Vanadio | 6 | 73 | 181 | 73 | 108 | 73 |
| Copper | Cu | Copper | 4 | 29 | 64 | 29 | 35 | 29 |
| Silver | Ag | Copper | 5 | 47 | 107 | 47 | 61 | 47 |
| Gold | Au | Copper | 6 | 79 | 196 | 79 | 118 | 79 |
Contenido relacionado
Sodium hydroxide
Mitochondrial crest
Electroanalytical method