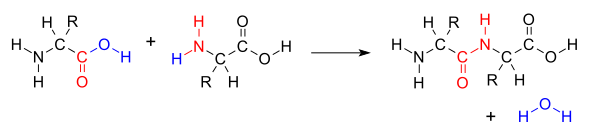
Peptide bond
The peptide bond is an amide bond between the amino group (–NH2) of an amino acid (AA) and the carboxyl group (–COOH) of another amino acid. Peptides and proteins are formed by the joining of amino acids by peptide bonds. Peptide bonding involves the formation of a CO-NH bond and the dehydration or loss of a water molecule (H2O), by losing the carboxyl group a hydrogen and an oxygen and the amino group a hydrogen. It is actually a substituted amide bond. The formation of this bond requires the input of energy, while its breaking (hydrolysis) releases it.
We can continue adding amino acids to the peptide, but always at the COOH terminus (in Figure 1, bottom right).
Naming the peptide begins with the NH2 terminus by agreement. If the first amino acid of our peptide were alanine and the second serine we would have the alanyl-serine peptide.
Structural characteristics of the link
In the 1940s and 1950s, studies based on X-ray diffraction on samples of amino acid, dipeptide, and tripeptide crystals by Linus Pauling and Robert Corey helped to understand the structure of the peptide bond, observing that:
- The C-N link that links to two amino acids is shorter than other C-N links
- The C-N link presents a partial double link as it stabilizes by resonance, which does not allow the turn on that link.
- The four atoms of the link (CO-NH) are in the same plane, with the Oxygen and Hydrogen in trans position. However, the rest of the links (N-C and C-C) are true simple links, with what might have turned (signed to in the image).
This rigid planar arrangement is the result of resonance stabilization of the peptide bond. For this reason, the framework of a peptide is constituted by the series of successive planes separated by substituted methylene groups in which there can be rotation. But not all turns are possible, which places important restrictions on the possible number of conformations a protein can adopt.
If we call "Φ" to the value of the angle that the N-C link can adopt, and "Ψ" to that of the C-C bond, there will only be a few values allowed for Φ and Ψ (see Ramachandran graph); and it will depend to a great extent on the size and characteristics of the successive R groups.
Degradation
The peptide bond can be broken by hydrolysis (adding water). In its presence it will break apart, releasing 8–16 kilojoules/mol (2–4 kcal/mol) of free energy. In nature this process is extremely slow (more than 1000 years), but there are ways to speed it up:
- Acid hydrolysis: It is based on protracted protein boiling with strong acid solutions (HCl and H2SO4). This method completely destroys the tryptophan and part of the seine and treonine.
- Basic hydrolysis: Respects amino acids that are destroyed by the previous hydrolysis. NaOH or Ba(OH)2 is normally used.
- Enzymatic hydrolysis: It is the most common form in living beings, proteolytic enzymes are used whose activity is slow and often incomplete, however no racemization occurs and amino acids are not destroyed; therefore it is very specific. The enzymes trypsin and quimotripsin, present in digestive juices, are examples of protein enzymes.
- Temperature hydrolysis: in normal conditions, high temperature does not destroy peptide bonds, although it can denaturalize the protein (rupture of secondary, tertiary and quaternary structures). However, extreme temperatures applied for a long time can come to destroy the peptide bonds (give 110 degrees 48 h).
Contenido relacionado
Fur
Niemann-Pick disease
Neurology