Ozone layer
The ozone layer is a layer that surrounds the Earth, preventing the sun's rays and ultraviolet rays from reaching living beings. The area of the Earth's stratosphere that contains a relatively high concentration of ozone is called the ozone layer or ozonosphere. This layer, which extends from approximately 15 km to 50 km in altitude, gathers 90% of the ozone present in the atmosphere and absorbs 97-99% of high-frequency ultraviolet radiation (150-300 nm). It was discovered by physicists Charles Fabry and Henri Buisson in 1913.
Its properties were examined in detail by British meteorologist G.M.B. Dobson, who developed a simple spectrophotometer that could be used to measure stratospheric ozone from the Earth's surface. Between 1928 and 1958 Dobson established a worldwide network of ozone monitoring stations, which continue to operate today. The Dobsonian unit, a unit of measurement for the amount of ozone, was named in his honor.
Origin of the ozone layer
Ozone is the alotropic form of oxygen, which is stable in certain pressure and temperature conditions. It is a gas composed of three oxygen atoms (O3{displaystyle O_{3}}).
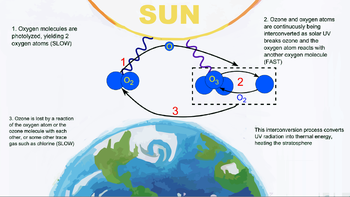
The photochemical mechanisms produced in the ozone layer were investigated by British physicist Sydney Chapman in 1930. The formation of ozone of the Earth's stratosphere is catalysed by the ultraviolet light photons that when interacting with the gaseous oxygen molecules, which are made up of two oxygen atoms (O2{displaystyle O_{2}}), separates them in the atoms of oxygen (atomic oxygen) constituent; atomic oxygen is combined with those molecules of oxygen O2{displaystyle O_{2}} that still remain without dissociating, thus forming ozone molecules, O3{displaystyle O_{3}}.
The ozone concentration is highest between 15 and 40 km, with a value of 2-8 particles per million, in the area known as the ozone layer. If all that ozone were compressed to the pressure of air at sea level, this layer would only be 3 millimeters thick.
Ozone acts as a filter, or protective shield, from harmful, high-energy radiation that reaches the Earth, allowing others such as long-wave ultraviolet to pass through, which thus reaches the surface. This ultraviolet radiation is what allows life on the planet, since it is what allows photosynthesis to take place in the plant kingdom, which is at the base of the trophic pyramid.
Apart from the ozone layer, the remaining 10% of ozone is contained in the troposphere, and it is dangerous for living beings due to its strong oxidizing nature. High concentrations of this compound at surface level form the so-called photochemical smog. The origin of this ozone is explained by 10% as coming from ozone transported from the stratosphere and the rest is created from various mechanisms, such as that produced by electrical storms that ionize the air and make it, very briefly, a good conductor. of electricity. Two consecutive lightning bolts can sometimes be seen following approximately the same path.
The dynamic equilibrium of ozone
Ozone is produced by the following reaction:
- O2+h.. → → O+O{displaystyle O_{2}+hnu rightarrow O+O}
- O+O2→ → O3{displaystyle O+O_{2}rightarrow O_{3}}}
That is, the molecular oxygen found in the upper layers of the atmosphere is bombarded by solar radiation. Of the wide spectrum of incident radiation, a certain fraction of photons meets the energy requirements necessary to catalyze the breaking of the double bond of the oxygen atoms of the molecular oxygen molecule.
Subsequently, the Sun converts an ozone molecule into a diatomic oxygen molecule and an unbonded oxygen atom:
- O3+h.. → → O2+O{displaystyle O_{3}+hnu rightarrow O_{2}+O}
During the dark phase (the night of a certain region of the planet), monatomic oxygen, which is highly reactive, combines with ozone from the ozonosphere to form a diatomic oxygen molecule:
- O3+O→ → 2O2{displaystyle O_{3}+Orightarrow 2O_{2}}}
To keep the ozone layer constant in the stratosphere, this photochemical reaction must occur in perfect balance, but these reactions are easily disturbed by molecules, such as chlorinated compounds (such as chlorofluorocarbons) and brominated compounds. On average, one chlorine atom is capable of destroying up to 100,000 ozone molecules, which is why small amounts can break down enough ozone to significantly deplete the ozone layer.
Ozone layer problems
The observational monitoring of the ozone layer, carried out in recent years, has reached the conclusion that this layer can be considered seriously threatened. This is the main reason why the General Assembly of the United Nations met on September 16, 1987, signing the Montreal Protocol. In 1994, the General Assembly of the United Nations proclaimed September 16 as the International Day for the Preservation of the Ozone Layer.
Serious depletion of the ozone layer will cause an increase in cases of melanomas, skin cancer, eye cataracts, suppression of the immune system in humans and other species. It will also affect crops sensitive to ultraviolet radiation.
To preserve the ozone layer, the use of chemical compounds such as chlorofluorocarbons (industrial refrigerants, propellants), and soil fungicides (such as methyl bromide) must be reduced to zero (Argentina, 900 tons/year) that They destroy the ozone layer at a rate 50 times higher than CFCs.
Hopes for a solution
The latest satellite measurements indicate that the hole in the ozone layer is shrinking, while levels of chlorofluorocarbons (CFCs) have decreased. These chemicals damage the ozone layer of the atmosphere that it protects our planet. For more than fifty years, the number of CFCs present in the upper atmosphere has increased steadily until the year 2000. Since then, the CFC concentration has decreased at the rate of almost 1% per year. The decline suggests that the hole in the ozone layer may close by mid-century. However, these products still cause damage. Despite the decline, the Antarctic hole in 2005 had reached an area of almost 29,000,000 km² (square kilometers), more than three times the size of Australia.
However, the recent Montreal Protocol Scientific Assessment Panel report, published in 2022, states that almost 99% of banned substances that affect the ozone layer have been phased out. The full recovery of the ozone layer is now expected in the next four decades.
Contenido relacionado
Annex: World Network of Biosphere Reserves
Matthiola fruticulosa
Deforestation