Oxidation state
In chemistry, the oxidation state (EO) is an indicator of the degree of oxidation of an atom that is part of a compound or other chemical species (for example, an ion). Formally, it is the hypothetical electrical charge that the atom would have if all its bonds with different elements were 100% ionic.
The EO is represented by numbers, which can be positive, negative or zero. In some cases, the average oxidation state of an element is a fraction, such as +8/3 for iron in magnetite (Fe3O4). The highest known EO is +8 for ruthenium tetraoxides, xenon, osmium, iridium, hassium, and some complex plutonium compounds, while the lowest known EO is -4 for some carbon group elements (group 14 elements).
According to IUPAC regulations, the symbol of the chemical element must be written as a superscript, indicating the number first and followed by the sign. For example Al3+
Oxidation occurs when an element or compound loses one or more electrons. Generally, when a substance is oxidized (loses electrons), another substance receives or captures these electrons, reducing itself. This is the basic mechanism that promotes oxidation-reduction or redox reactions.
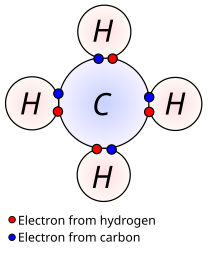
An atom tends to obey the octet rule in order to have an electronic configuration equal to that of noble gases, which are very chemically stable (their atoms form chemical bonds with almost no one, not even with themselves). This rule holds that an atom tends to have eight electrons in its outermost energy level. In the case of hydrogen, it tends to have 2 electrons, which would give it the same electronic configuration as that of helium.
When an atom A needs, for example, 3 electrons to obey the octet rule, then said atom tends to have an oxidation number of 3-, when it acquires those 3 electrons. On the other hand, when an atom B has the 3 electrons that must be given up for atom A to fulfill the octet law, then this atom tends to have an oxidation number of 3+, when it gives up those 3 electrons. In this example we can deduce that atoms A and B can unite to form a compound, and that this depends on the interactions between them. The octet and duet rules can be satisfied by sharing electrons (forming covalent compounds, for example in molecules such as water) or giving up and acquiring electrons (forming ionic compounds such as sodium chloride crystals).
Chemical elements are divided into 3 large groups, classified by the type of electrical charge they can acquire when participating in a chemical reaction:
- Metals.
- No metals.
- Noble gases.
There are metallic elements that, depending on the conditions to which they are subjected, can function interchangeably as metals or non-metals. These elements are called semimetals or metalloids.
Metallic elements (which tend to donate electrons) when they form compounds normally have positive oxidation states. Non-metallic and semi-metallic elements, on the other hand, can have positive and negative oxidation states, depending on the compound they are constituting.
Examples
- Sodium chloride
2Na0 + Cl02 → 2Na1+ + 2Cl1-
Gases of only one type of element, in this case chlorine, are present in diatomic form.
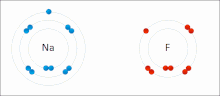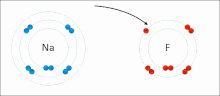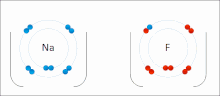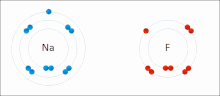
Sodium (Na) combines with chlorine (Cl), producing sodium chloride. The oxidation number of both uncombined elements is 0 (zero), since they are electrically balanced. The oxidation number of combined sodium is 1+, since it loses an electron. The oxidation number of combined chlorine is 1-, since it accepts the electron donated by sodium.
- Aluminum oxide
Al0 + O02 → Al3+ + 2O2−
Oxygen (O) is present in diatomic (gas) form.
Aluminum (Al) combines with oxygen (O), producing aluminum oxide (Al2O3). The oxidation number of both uncombined elements is 0 (zero), since they are electrically balanced. The oxidation number of combined aluminum is 3+, since it gives up three electrons. The oxidation number of combined oxygen is 2−, since it accepts up to 2 electrons.
The electrons given up and accepted by the different elements create a problem with electrical charges. For example, aluminum gives up three electrons and oxygen only accepts two, so one is left over. From this it is concluded that not a single oxygen atom intervenes in the reaction, so we proceed to balance the equation, so that all the transferred electrons coincide with the capacities of each acceptor element.
The balanced equation looks like this:
4Al0 + 3O02 → 4Al3+ + 6O2− → 2Al3+ + 3O2−
With which the perfect balance is achieved so that all the excess electrons are accommodated. Elements in a free or ground state element have an oxidation number equal to 0.
- All metallic elements (which yield electrons) when forming compounds generally have positive oxidation states.
- Non-metallic and non-metallic elements may have positive and negative oxidation states, depending on the compound they are forming.
- For any element the maximum oxidation states is the corresponding to the group number.
- The minimum possible oxidation condition of an element is 4−, and some of the elements of the 4A group have it.
- Non-metals have a unique negative oxidation state, which is equal to the group number minus 8.
- The elements of the 1A and 2A groups possess the 1+ and 2+ oxidation states respectively.
- Hydrogen works with oxidation condition 1+ generally, except when it forms metallic spindles where its oxidation condition is 1−.
- The O oxidation number is 2−, when it forms peroxides, where it is 2−, and when it forms superoxide, where it is 1−.
- The sum of the oxidation states of all the elements of a compound is equal to its net load.
Rules for oxidation states
- The oxidation status of all elements in free state, not combined with others, is zero (e.g., Na, Cu, Mg, H2O,2Cl2, N2).
- The oxidation condition of the H is 1+, except in the metal spins, in which it is 1- (e.g., NaH, CaH2).
- The oxidation condition of the O is 2-, except in the peroxides in which it is -1, in the superoxides that is 1/2- and in the oxygen fluoride (OF)2), where it is 2+.
- The oxidation status of the metal element of an ionic compound is positive.
- In covalent compounds, the number of negative oxidation is assigned to the most electro-negative atom and all others are positive.
- The algebraic sum of the oxidation states of the elements of a compound is zero.
- The algebraic sum of the oxidation states of the elements of a polyatomic ion is equal to the ion load.
Contenido relacionado
Gunpowder
Cysteine
Trace element