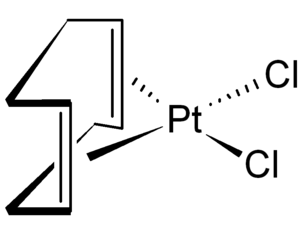
Organometallic compound
An organometallic compound is a compound in which the carbon atoms of an organic ligand form covalent bonds with a metal atom. Compounds based on chains and rings of carbon atoms are called organic, and this is the basis for the name organometallic. The characteristic of these compounds is the presence of bonds between metal and carbon atoms (which can be single, double or triple) and therefore those compounds in which a metal is attached to a molecule or fragment by an atom are not considered organometallic. other than carbon, as occurs in some coordination compounds. This group includes a large number of compounds and is considered by some chemists as a separate group from organic and inorganic compounds.
Formally, organometallic compounds are those that have, directly, bonds between metal atoms (or metalloids) and carbon atoms, M+δ–C-δ, of greater or lesser polarity. That is, a compound is considered organometallic if it contains at least one carbon-metal bond. In this context the suffix "metallic" is broadly interpreted to include both some non-metals (such as phosphorus) and metalloids such as B, Si and As as well as true metals. This is because in many cases the chemistry of the elements B, Si, P, and As resembles the chemistry of the respective metal counterparts. Therefore, the term organometallic compounds is also occasionally used to include the aforementioned nonmetals and semimetals. In all cases, these are elements that are less electronegative than carbon.
Type of bond and stability of organometallic compounds
In addition to the metal-carbon bond, the metal can be attached to other ligands forming metal-ligand units. The fundamental type of bond between carbon and metal is covalent, but there are also compounds with two-center, two-electron bonds. In addition, in organometallic compounds with transition metals it is important to consider the 18-electron rule.
Until 1940, many organometallics could not be synthesized, so it was assumed that the carbon-metal bond was not very stable. Actually, it is not a thermodynamic cause that causes the difficulty of its synthesis, but a kinetic problem that makes it necessary to inhibit the decomposition reactions of the compounds formed. However, thermodynamic stability decreases with the size of the metal.
As the temperature increases, many of them decompose, but sometimes they are slow reactions, so it is possible to synthesize these compounds. In general, they are not very stable against oxidation and hydrolysis, since in both cases, products of great stability.
Synthesis of organometallic compounds
Synthesis methods are very varied:
- Reactions between a metal and an organic halure
- Metal exchange reactions, between a metal and an organometallic compound of another metal.
- Reactions of an organometallic compound with a metal halur.
- Insertion reactions:
- Insertion of olefins and acetylenes in metal-hydrogen links for metals of groups 13 and 14.
- Insertion reactions for the formation of metal-carbon bonds for metals from other groups.
- Diazo compound reactions.
- Decarboxylation reactions of metal salts.
- Reactions for the formation of organometallic mercury and talium with aromatic compounds.
- Training reactions of mercury organometallics with olefins and acetylenes.
Types of organometallic compounds
Different classifications can be established.
According to the metal-carbon bond
If we consider the character of the bond between the metal (or semi-metal) and the carbon we will have:
- Ionic (non-molecular): Organometallic compounds of sodium, potassium, blond, cesium, calcium, strontium, barium and lanthanides.
- Intermediate: Organometallic compounds of lithium, berylium, magnesium and aluminum. They have electron deficits and tendency to form alkylo bridges and multicentered links.
- Covalentes (moleculares): Organometallic compounds of boro, silicon and elements of groups 12 to 16 that are below the third period, such as Zn, Cd, Hg, Ga, In, Tl, Ge, Sn, Pb, As, Sb, Bi, Se, Te and Po.
- Complexes: Organometallic compounds of transition metals from groups 3 to 11, in which pi-type links abound (π).
Depending on the type of ligand
- Tipping that bind to metal through a single carbon atom.
- Related alkylos and ligands: the metal-carbon link, M-C, is simple.
- Alquenyls and acile: the metal-carbon link, M-C, is double.
- Alquinilos and cyanides: the metal-carbon link, M-C, is triple.
- Tipping that bind to metal through several carbon atoms.
The number of carbon atoms attached directly to the metal is symbolized by ηn, read n-hapto.
Depending on the group to which the metal or metalloid belongs
- Organometallic compounds of group 1:
- Organolithium compounds.
- Organyls of other alkaline metals: organosodium, organopotasy, organorubidium and organocesio.
- Organizational compositions of groups 2 and 12:
- Organometallic compounds of group 2:
- Organoberilio compounds.
- Organomagnesio compounds.
- Organometallic compounds of calcium, strontium and barium.
- Organometallic compounds of group 12:
- Organozinc compounds.
- Organizational compounds.
- Organomercury compounds.
- Organometallic compounds of group 2:
- Organizational compositions of Group 13:
- Organoboro compounds.
- Organoaluminium compounds.
- Organometallic compounds of Galio, Indio and Talio:
- Organilos σ de Ga, In and Tl and their aducts.
- Complex π de Ga, In and Tl.
- Organizational compositions of Group 14:
- Organosilicon compounds: organilos, methyl chlorrosylans and silicone.
- Organogermanium compounds.
- Organizational compounds.
- Organoplome compounds.
- Organometallic compounds of Group 15: organilos of Arsenico, Antimony and Bismuto.
- Organometallic compounds of transition metals.
- With bad ligands.
- Alkylos and arilos of transition metals.
- Organometallic complexes sigma.
- With ligandos dadores σ / acceptres π.
- Transition metals.
- Transition metal alquinilos.
- Complex transition metals.
- Complex carbinos of transition metals.
- Metallic carbonyls, metallic carbonyl complexes and metallic carbonyl hydrochlors.
- Carbonyl metal halours.
- With ligandos dadores σ, π /aceptores π.
- Oil complexes: homoalquenos and heteroalquenos.
- Complex alkynos: homoalquinos and heteroalquinos.
- Complex alilos and enilos. Trienyl and dienyl complexes. Cyclopentadienival complexes.
- Complex metal earrings.
- With bad ligands.
- Organometallic compounds of lanthanides and actinides
Applications of organometallic complexes.
Every day there are more abundant industrial and laboratory applications of these complexes. Among other uses, we can cite:
- Catalytic reactions.
- Oil isomerization reactions.
- Railation and vinylation reactions (Heck reaction).
- Metathesis of alchenos.
- Reactions of oligomerization and polymerization.
- Oxidation of olefins (Wacker Process).
- Alk hydrogenation.
- Fischer-Tropsch reactions.
- Water gas displacement reaction.
- Monsanto process for acetic acid synthesis.
- Olefin hydroformilation (Oxo Process).
- Olefin hydrocynation.
- Carbonylation of olefins (Reaction of Reppe).
- Activation of C-H links in alcanos.
Chemical bonds of carbon with other atoms
| CH | He | |||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | |||||||||||
| CNa | CMg | CAl | CSI | COP | CS | CCl | CAr | |||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CC | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr | |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CI | CSn | CSb | CTe | CI | CXe | |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | COP | CAt | Rn | ||
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||
| ↓ | ||||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYB | CLu | ||||
| Ac | CTh | COPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No. | Lr | ||||
| Basic organic chemistry. | Many uses in Chemistry. |
| Academic research, but not a wide use. | Link unknown / not evaluated. |
Contenido relacionado
Heat capacity
Fusion energy
Inverse osmosis