Organochlorine
  |
| Two representations of an organochlorine: chloroform. |
An organochlorine compound, chlorinated hydrocarbon, chlorocarbon or chlorinated organic compound is an organic chemical compound, that is, composed of a skeleton of carbon atoms, in which some of the hydrogen atoms attached to carbon have been replaced by chlorine atoms, attached by covalent bonds to carbon.
Its wide structural variety and divergent physical properties lead to a wide range of applications. Many chlorinated derivatives are controversial due to the effects of these compounds on the environment and human and animal health, being generally harmful to living beings, and may even be carcinogenic. Many of them are used for their insecticidal or pesticide action; others are by-products of the industry.
Some examples of organochlorines are: trichloromethane CHCl3, carbon tetrachloride CCl4, DDT or sucralose.
Physical properties
Substituent chlorine atoms modify the physical properties of organic compounds in various ways. They are usually denser than water, due to the high atomic mass of chlorine. These chlorine atoms induce stronger intermolecular interactions than when they have hydrogen atoms. The effect is illustrated by the evolution of boiling points: methane (-161.6 °C), methyl chloride (-24.2 °C), dichloromethane (40 °C), chloroform (61.2 °C), and carbon tetrachloride (76.72 °C). The increase in intermolecular interactions is attributed to both the effects of Van der Waals forces and the increased polarity of the bonds.
Presence in nature
Although rarer than non-halogenated organic compounds, many organochlorine compounds have been isolated from natural sources ranging from bacteria to humans. Chlorinated organic compounds are found in almost all classes of biomolecules, including including alkaloids, terpenes, amino acids, flavonoids, steroids, and fatty acids. Organochlorines, including dioxins, are produced in high-temperature environments such as forest fires. Dioxins have been found in preserved ash from fires ignited by lightning prior to the production of synthetic dioxins. In addition, many simple chlorinated hydrocarbons such as dichloromethane, chloroform, and carbon tetrachloride have been isolated from marine algae. Most part of the chloromethane present in the environment is produced naturally by decomposition of biological remains, forest fires and volcanoes. The natural organochlorine epibatidine, an alkaloid isolated from frogs, has a potent analgesic effect and has stimulated research into new pain medications.
Preparation
From chlorine
Alkanes and arylalkanes can be chlorinated under radical-free conditions, with UV light. However, the degree of chlorination is difficult to control. Aryl chlorides can be prepared by Friedel-Crafts halogenation, using chlorine and a Lewis acid as catalyst.
The haloform reaction, using chlorine and sodium hydroxide, is also capable of generating alkyl halides from methyl ketones and related compounds. Chloroform was formerly produced in this way.
Chlorine undergoes addition reactions to multiple bonds as in alkenes, alkynes and others, giving di, tri or tetra-chlorinated compounds.
Reaction with hydrogen chloride
Alkenes react with hydrogen chloride (HCl) to give alkyl chlorides. For example, the industrial production of chloroethane comes from the reaction of ethylene with HCl:
- H2C=CH2 + HCl → CH3CH2Cl
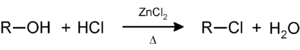
Secondary and tertiary alcohols react with Lucas reagent (zinc chloride) in concentrated hydrochloric acid to obtain the corresponding alkyl halide, so this reaction serves as a method to classify alcohols:
Other chlorinating agents
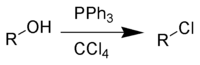
In the laboratory, alkyl chlorides are most easily prepared by reacting alcohols with thionyl chloride (SOCl2), phosphorous trichloride (PCl3), or phosphorus pentachloride (PCl5):
- ROH + SOCl2 → RCl + SO2 + HCl
- 3 ROH + PCl3 → 3 RCl + H3PO3
- ROH + PCl5 → RCl + POCl3
In the laboratory, the use of thionyl chloride is very convenient, since the by-products are gaseous.
On the other hand, Apple's reaction:
Reactions
Alkyl chlorides are versatile building blocks in organic chemistry. While alkyl bromides and iodides are more reactive, alkyl chlorides tend to be cheaper and easier to obtain. Alkyl chlorides are easily attacked by nucleophiles.
- Heating alkylo halogenides with sodium hydroxide or water are obtained alcohols.
- Reactions with alcoxides or oxides produce ethers in the synthesis of Williamson ethers
- The reaction with tioles give typoeters.
- Alkyl chlorides easily react with amines to give replaced amines.
- Alkyl chlorides are replaced by other softer halures such as iodine in Finkelstein's reaction.
- The reaction with other pseudohalides, such as azides, cyanides and tiocianates, are also possible.
- In the presence of a strong base, alkyl chlorides suffer from dehydrohalogenation to give alchenos or alchenos.
- Alkyle chlorides react with magnesium to give Grignard reagents, transforming an electrophilic compound into a nucleophilic compound.
- The reaction of Wurtz reductively links the radicals of two alkylo halogenides while the haluro joins with sodium.
Applications
Vinyl Chloride
The largest application of organochlorines in chemistry is the production of vinyl chloride. Annual production in 1985 was around 18 million tons rising in 2005 to over 32 million tons, almost all of which was converted to polyvinyl chloride (PVC).
Chloromethanes
Most low molecular weight chlorinated hydrocarbons such as chloroform, dichloromethane, dichloroethene and trichloroethane are useful solvents. These solvents tend to be relatively nonpolar, making them immiscible with water and effective in cleaning applications such as degreasing and dry cleaning. Several billion kilograms of chloromethanes are produced annually, mainly by chlorination of methane:
- CH4 + x Cl2 → CH4-xClx + x HCl
The most important is dichloromethane, which is used mainly as a solvent. Chloromethane is a precursor to chlorosilanes and silicones. Of historical importance, but on a smaller scale today, we have chloroform, used primarily as a precursor to chlorodifluoromethane (CHClF2) and tetrafluoroethene used in the manufacture of Teflon.
Pesticides
Many pesticides contain chlorine. Some notable examples are: DDT, dicofol, heptachlor, endosulfan, chlordane, aldrin, dieldrin, endrin, lindane, mirex, and pentachlorophenol. These can be hydrophilic or hydrophobic depending on their molecular structure. Many of these agents have been banned in various countries, for example, mirex and aldrin.
Insulators
Polychlorinated biphenyls (PCBs) were once in common use as electrical insulators and heat transfer agents. Its use has generally been phased out due to health concerns. PCBs have been replaced by polybrominated diphenyl ethers (PBDEs), which have similar toxicity and bioaccumulation problems.
Toxicity
Some types of organochlorine compounds have significant toxicity to plants or animals, including humans. Dioxins are produced when organic matter is burned in the presence of chlorine, and some insecticides such as DDT are persistent organic pollutants (POPs), which pose a hazard when released into the environment. For example, DDT, which was widely used to control insect pests in the mid-XX century, also accumulates in food chains, and causes reproductive problems (such as eggshell thinning) in certain bird species.
When chlorinated solvents, such as carbon tetrachloride, are not properly removed, they accumulate in groundwater. Some highly reactive organochlorine compounds such as phosgene have been used as chemical warfare agents.
However, the presence of chlorine in an organic compound does not guarantee its toxicity. Many organochlorines are safe enough to be consumed in food and medicine. For example, peas and lima beans contain natural plant hormones chlorinated 4-chloroindole-3-acetic acid (4-Cl-IAA); and the sweetener sucralose (Splenda) is widely used in dietetic products. As of 2004, there were at least 165 organochlorine compounds approved for use as drugs worldwide, including the natural antibiotic vancomycin, the antihistamine loratadine (Claritin), the antidepressant sertraline (Zoloft), the antiepileptics lamotrigine (Lamictal), and the inhalation anesthetic isoflurane.
Rachel Carson brought the issue of the toxicity of the pesticide DDT to the public eye with her 1962 book Silent spring. Although many countries have eliminated the use of some types of organochlorine compounds, such as the US ban on DDT, persistent DDT, PCBs, and other organochlorine residues are still found in humans and mammals throughout the world. the planet many years after its production and use have been limited. In areas of the Arctic, particularly high levels are found in marine mammals. These chemicals are concentrated in mammals, and are even found in human breast milk. Males of these species tend to have much higher levels, as females reduce their concentration upon transfer to their offspring through breastfeeding.
Chemical bonds of carbon with other atoms
| CH | He | |||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | |||||||||||
| CNa | CMg | CAl | CSI | COP | CS | CCl | CAr | |||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CC | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr | |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CI | CSn | CSb | CTe | CI | CXe | |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | COP | CAt | Rn | ||
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||
| ↓ | ||||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYB | CLu | ||||
| Ac | CTh | COPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No. | Lr | ||||
| Basic organic chemistry. | Many uses in Chemistry. |
| Academic research, but not a wide use. | Link unknown / not evaluated. |
Contenido relacionado
Common cold
Pollakiuria
Legionellosis