Organic chemistry
Organic chemistry is the branch of chemistry that studies a large class of molecules that mostly contain carbon forming covalent bonds: carbon-carbon or carbon-hydrogen and other heteroatoms, also known as as organic compounds.
Due to the omnipresence of carbon in the compounds that this branch of chemistry studies, this discipline is also called carbon chemistry.
History
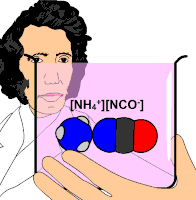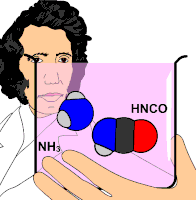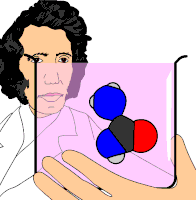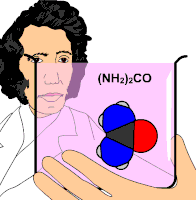
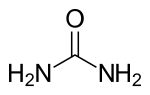
Organic chemistry was established or instituted as a discipline in the 1930s. The development of new methods of analysis of substances of animal and vegetable origin, based on the use of solvents, such as ether or alcohol, allowed the isolation of a large number of organic substances that received the name &# 34;immediate principles". The advent of organic chemistry is often associated with the discovery, in 1828, by the German chemist Friedrich Wöhler, that the inorganic substance ammonium cyanate could be converted to urea, an organic substance found in the urine of many animals. Before this discovery, chemists believed that to synthesize organic substances, the intervention of what they called 'the vital force', that is, living organisms, was necessary. Wöhler's experiment broke the barrier between organic and inorganic substances. In this way, modern chemists consider organic compounds to be those that contain carbon and hydrogen, and other elements (which can be one or more), the most common being: oxygen, nitrogen, sulfur, and halogens.
In 1856, Sir William Henry Perkin, while trying to study quinine, accidentally manufactured the first organic dye now known as Perkin's mallow.
The difference between organic chemistry and biological chemistry is that in the second the DNA molecules have a history and, therefore, in their structure they tell us about their history, about the past in which they have been constituted, while that an organic molecule, created today, is only a witness of its present, without past and without historical evolution.
Timeline
- 1675: Lémery classifies natural chemicals, according to their origin in minerals, vegetables and animals
- 1784: Antoine Lavoisier demonstrates that all plant and animal products are basically made up of carbon and hydrogen and, to a lesser extent, nitrogen, oxygen and sulfur
- 1807: Jöns Jacob Berzelius classifies chemicals in:
- Professional: those who come from living organisms.
- Inorganic: those who come from the raw material.
- 1816: Michel Eugène Chevreul prepares different soaps from different sources of fatty acids and various bases, thus producing different salts of fatty acids (or soaps), which were nothing more than new organic products derived from natural products (grease animals and vegetables).
- 1828: Friedrich Wöhler, from inorganic substances and with normal laboratory techniques, synthesized the urea substance, the second artificially obtained organic substance, after the ammonia oxalate.
- 1856: Sir William Perkin synthesizes the first organic colorant by accident.
- 1865: August Kekulé proposed that the carbon atoms that form the benzene be joined in forming closed chains or rings.
First compendiums
The task of presenting organic chemistry in a systematic and global way was carried out through a publication that originated in Germany, founded by the chemist Friedrich Konrad Beilstein (1838-1906). His Handbuch der organischen Chemie (Handbook of Organic Chemistry) began publication in Hamburg in 1880 and consisted of two volumes collecting information on some fifteen thousand known organic compounds. When the Deutsche chemische Gesellschaft (German Chemical Society) tried to produce the fourth edition, in the second decade of the 20th century, the number of organic compounds had increased tenfold. Thirty-seven volumes were required for the basic edition, which appeared between 1916 and 1937. A 27-volume supplement was published in 1938, collecting information that appeared between 1910 and 1919.
Currently, the Fünftes Ergänzungswerk (fifth complementary series) is being published, which includes the documentation published between 1960 and 1979. In order to offer his latest works more quickly, the Beilstein The Institut has created the Beilstein On line service, which has been running since 1988. Recently, a CD-ROM, Beilstein Current Facts in Chemistry, has been regularly published., which selects chemical information from major journals. Currently, the aforementioned information is available through the Internet.
The soul of organic chemistry: carbon
The large number of organic compounds that exist can be explained by the characteristics of the carbon atom, which has four electrons in its valence shell: according to the octet rule it needs eight to complete it, so it forms four bonds (valence = 4) with other atoms. This special electronic configuration gives rise to a variety of possibilities for orbital hybridization of the carbon atom (chemical hybridization).
The simplest organic molecule that exists is methane. In this molecule, the carbon is sp3 hybridized, with the hydrogen atoms forming a tetrahedron.
Carbon forms covalent bonds easily to reach a stable configuration, these bonds are easily formed with other carbons, which allows frequently forming open (linear or branched) and closed (rings) chains.
Classification of organic compounds
The classification of organic compounds can be done in various ways: according to their origin (natural or synthetic), their structure (eg: aliphatic or aromatic), their functionality (eg: alcohols or ketones), or to their molecular weight (eg, monomers or polymers).
Classification according to their origin
The classification of organic compounds according to their origin is of two types: natural or synthetic. Often, those of natural origin are understood to be those present in living beings, but this is not always the case, since some organic molecules are also synthesized ex-vivo, that is, in inert environments, such as For example, formic acid in Comet Halle-Bopp.
Natural
In vivo
Organic compounds present in living beings or "biosynthesized" They constitute a large family of organic compounds. Your study has interest in medicine, pharmacy, perfumery, cooking and many other fields.
Carbohydrates
Carbohydrates are made primarily of carbon (C), oxygen (O), and hydrogen (H). They are often called "sugars", but this nomenclature is not entirely correct. They have a great presence in the plant kingdom (fructose, cellulose, starch, alginates), but also in the animal (glycogen, glucose). They are usually classified according to their degree of polymerization in:
- Monosaccharides (glucosa, fructosa, ribosa and desoxirribosa)
- Disaccharides (sacarous, lactose, maltosa)
- Trisaccharides (maltotrious, raffin)
- Polysaccharides (alginates, alkylated acid, cellulose, starch, etc.)
Lipids
The lipids are a group of organic molecules, most of them biomolecules, composed mainly of carbon and hydrogen and to a lesser extent oxygen, although they may also contain phosphorus, sulfur and nitrogen. Their main characteristic is being hydrophobic (insoluble in water) and soluble in organic solvents such as benzine, benzene and chloroform. In colloquial usage, lipids are incorrectly called fats, since fats are just one type of lipid from animals. Lipids fulfill various functions in living organisms, including energy reserves (such as triglycerides), structural (such as bilayer phospholipids), and regulatory (such as steroid hormones).
Protein
Proteins are polypeptides, that is, they are formed by the polymerization of peptides, and these by the union of amino acids. They can thus be considered "natural polyamides", since the peptide bond is analogous to the amide bond. They comprise a very important family of molecules in living beings, but especially in the animal kingdom. On the other hand, they are the product of the expression of genes contained in the DNA. Some examples of proteins are collagen, fibroins, or spider silk.
Nucleic Acids
Nucleic acids are polymers formed by repeating monomers called nucleotides, linked by phosphodiester bonds. Thus, long chains are formed; some nucleic acid molecules reach gigantic molecular weights, with millions of nucleotides chained together. They are formed by the molecules of carbon, hydrogen, oxygen, nitrogen and phosphate. Nucleic acids store the genetic information of living organisms and are responsible for hereditary transmission. There are two basic types, DNA and RNA.
Small Molecules
Small molecules are organic compounds of moderate molecular weight (generally considered "small" those with a molecular weight less than 1000 g/mol) and that appear in small amounts in living beings, but not by therefore its importance is less. They belong to different groups of hormones such as testosterone, estrogen or other groups such as alkaloids. Small molecules are of great interest in the pharmaceutical industry due to their relevance in the field of medicine.
Ex-vivo
They are organic compounds that have been synthesized without the intervention of any living being, in extracellular and extraviral environments.
Geological processes
Petroleum is a substance classified as a mineral in which a large amount of organic compounds are present. Many of them, such as benzene, are used by man as is, but many others are treated or derived to obtain a large amount of organic compounds, such as monomers for the synthesis of polymeric or plastic materials.
Atmospheric processes
The climate system is made up of the atmosphere, hydrosphere, biosphere, geosphere, and their interactions. Variations in the climatic balance can generate various processes such as global warming, the greenhouse effect or the depletion of the ozone layer.
Planetary synthesis processes
In the year 2000 formic acid, a simple organic compound, was also found in the tail of Comet Hale-Bopp. Since the organic synthesis of these molecules is infeasible under space conditions, this finding seems to suggest that the formation of the solar system must have been preceded by a period of warming during its final collapse.
Synthetic
Since Wöhler's synthesis of urea, a very high number of organic compounds have been chemically synthesized for human benefit. These include drugs, deodorants, perfumes, detergents, soaps, synthetic textile fibers, plastic materials, polymers in general, or organic dyes.
Simple hydrocarbon chains
Hydrocarbons
The simplest compound is methane, a carbon atom with four hydrogen (valence = 1), but the carbon-carbon union can also occur, forming chains of different types, since single, double or triples. When the rest of the links in these chains are with hydrogen, we speak of hydrocarbons, which can be:
- Saturated: with simple covalents, alcanos.
- Unsaturated: with double covalents (alquenos) or triple (alkynos).
- Cyclical hydrocarbons: Saturated hydrocarbons with closed chain, such as the cyclohexane.
- Aromatics: cyclical structure.
Radicals and Chain Branches
Radicals are fragments of carbon chains that hang from the main chain. Its nomenclature is made with the corresponding root (in the case of one carbon met-, two carbons et-, three carbons prop-, four carbons but-, five carbons pent-, six carbons hex-, and so on) and the suffix -il. In addition, the position they occupy is indicated with a number, placed in front. The simplest compound that can be made with radicals is 2-methylbutane. If there is more than one radical, they will be named in alphabetical order of the roots. For example, 2-ethyl, 5-methyl, 8-butyl, 10-docosene.
Classification according to functional groups
Organic compounds can also contain other elements, also other groups of atoms besides carbon and hydrogen, called functional groups. An example is the hydroxyl group, which forms alcohols: an oxygen atom bonded to a hydrogen atom (-OH), which has a free valence left. Likewise, there are also alkene functions (double bonds), ethers, esters, aldehydes, ketones, carboxylics, carbamoyls, azo, nitro or sulfoxide, among others.
Oxygenates
They are carbon chains with one or more oxygen atoms. They may be:
- Alcohols: The physical properties of an alcohol are mainly based on its structure. Alcohol is made up of an alcano and water. It contains a hydrophobic group (without water affinity) of the type of an alcano, and a hydroxyl group that is hydrophilic (with water affinity), similar to water. Of these two structural units, the group –OH gives alcohols its characteristic physical properties, and the alkylo is the one that modifies them, depending on their size and shape.
The –OH group is very polar and, what is more important, it is capable of establishing hydrogen bonds: with its partner molecules or with other neutral molecules.
Depending on the number of -OH groups that make up the alcohol, it can be classified as monohydroxylated (presence of one hydroxyl) or polyhydroxylated (two or more hydroxyl groups in the molecule).
- Aldehydes: Aldehydes are organic compounds characterized by having the functional group -CHO. They are referred to as the corresponding alcohols, changing the -ol termination by -al: that is, the H-C=O carbonyl group is attached to a single organic radical.
- Cetonas: A ketone is an organic compound characterized by having a functional carbonyl group attached to two carbon atoms, unlike an aldehyde, where the carbonyl group is attached at least to a hydrogen atom. When the functional carbonyl group is the most relevant in this organic compound, the cetons are named adding suffix -one to the hydrocarbon from which they come (hexane, hexanona; heptano, heptanona; etc). It can also be named after blinding the radicals to which it is united (e.g. methylphenyl cetona). When the carbonyl group is not the priority group, the oxo prefix is used (example: 2-oxopropanal). The carbonyl functional group consists of a carbon atom combined with a double covalent bond to an oxygen atom. Having two carbon atoms attached to the carbonyl group, is what differentiates it from carboxylic acids, aldehydes, esters. The double link with oxygen is what differentiates it from alcohols and ethers. Cetons are usually less reactive than aldehydes since alkylic groups act as electron damages by inductive effect.
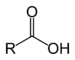
- Carboxylic acids: Carboxylic acids constitute a group of compounds that are characterized because they have a functional group called carboxyl group or carboxi group (–COOH); a hydroxyl group (-OH) and carbonyl (C=O) are produced when they match the same carbon. It can be represented as COOH or CO2H...
- Esters: The esters present the ester group (-O-CO-) in their structure. Examples of substances with this group include salicylic acetyl acid, a component of aspirin, or some aromatic compounds such as isoamile acetate, with characteristic banana smell. Oils are also fatty acid esters with glycerol.
- Ethers: Ethers present the ether(-O-) group in their structure. They usually have low boiling point and are easily decomponible. For both reasons, low molecular mass ethers are often dangerous as their vapors can be explosive.
Nitrogenous
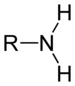
- Aminas: Amines are organic compounds characterized by the presence of the amine group (-N. Amines can be primary (R-NH)2, secondary (R-NH-R") or tertiary (R-NR'-R"). Amines usually give slightly yellowish compounds and smells that remind fish or urine.
- Amidas: Amidas are organic compounds characterized by the presence of the group amida (-NH-CO-) in its structure. Proteins or polypeptides are natural polyamides formed by peptide bonds between different amino acids.
- Isocianates: Isocianates have the issociate group (-N=C=O). This group is very electrolyte, reacting easily with water to decompose by Hofmann's transposition to give a coalic amine and anhydric, with hydroxyls to give urethanes, and with primary or secondary amines to give ureas.
Cyclics
They are compounds that contain a saturated cycle. An example of these are norbornanes, which are actually bicyclic compounds, terpenes, or hormones such as estrogen, progesterone, testosterone, or other biomolecules such as cholesterol.
Aromatics
Aromatic compounds have cyclic unsaturated structures. Benzene is the clear example of an aromatic compound, among whose derivatives are toluene, phenol or benzoic acid. In general, an aromatic compound is defined as one that has rings that comply with Hückel's rule, that is, they have 4n+2 electrons in π orbitals (n=0,1,2,...). Organic compounds that have a group other than carbon in their rings (usually N, O, or S) are called heterocyclic aromatic compounds. Thus aromatic compounds are usually divided into:
- Benzene derivatives: Polycyclics (anthracine, naphthalene, fenantreno, etc.), phenols, aromatic amines, fulerenos, etc.
- Heterocyclic compounds: Piridina, furano, thiophoeno, pirrol, porfirina, etc.
Isomers
Since carbon can be bonded in different ways, a chain can have different bond configurations giving rise to so-called isomers, molecules that have the same chemical formula, but different structures and properties.
There are different types of isomerism: chain isomerism, function isomerism, tautomerism, stereoisomerism, and configurational stereoisomerism.
The example shown on the left is a case of chain isometry in which the compound with formula C6H12 can be a cyclo (cyclohexane) or a linear alkene, 1-hexene. An example of function isomerism would be the case of propanal and acetone, both with the formula C3H6O.
Organic compounds
Organic compounds can be divided very broadly into:
- Alphatic compounds
- aromatic compounds
- Heterocyclic compounds
- Organizational compositions
- Polymers
Relation to biology
One of the main relationships between organic chemistry and biology is the study of the synthesis and structure of organic molecules of importance in the molecular processes carried out by living organisms, that is, in metabolism. Biochemistry is the field interdisciplinary scientist who studies living things, and since they use compounds that contain carbon, organic chemistry is essential to understand metabolic processes.
In biological terms, organic chemistry is of great importance, especially in a cellular context, and we can exemplify this with molecules such as carbohydrates, present from the plasmatic membrane as well as in the chemical structure of DNA, lipids, which are the base main part of the plasma membrane, the proteins that help support an organism or its functions as enzymes and DNA, a molecule in charge of protecting the genetic information of living organisms.
Contenido relacionado
Alpha particle
Lanthanides
Bronze