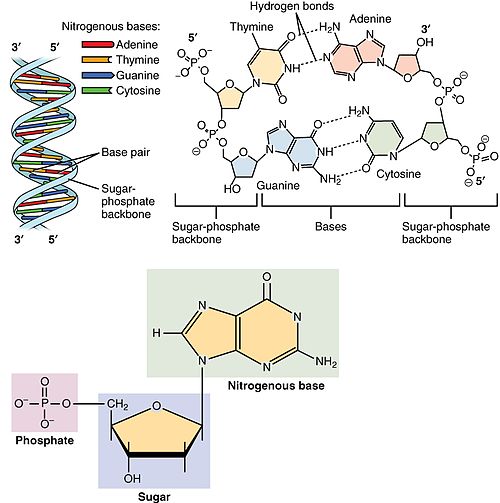
Nucleotide
green background (right top) nitrogen base.
blue background (low center) pentosa sugar (in the form of pentagon).
pink background (low left) phosphate.
Nucleotides are small molecules synthesized by all living organisms, which are formed by the union of three elements: a nitrogenous base, a simple sugar and a phosphate group.
Nucleotides are the basic structural components (monomers) of nucleic acids, in which they are located as the transverse rungs of the unwound form of the nucleic acid ladder, but also perform important functions as a free molecule (for example, ATP or GTP).
History
In 1919, the biochemist P. Levene identified that a nucleotide was made up of a nitrogenous base, a sugar, and a phosphate. Levene suggested that DNA generated a solenoid (spring)-shaped structure, with units of nucleotides attached to through the phosphate groups.
Structure
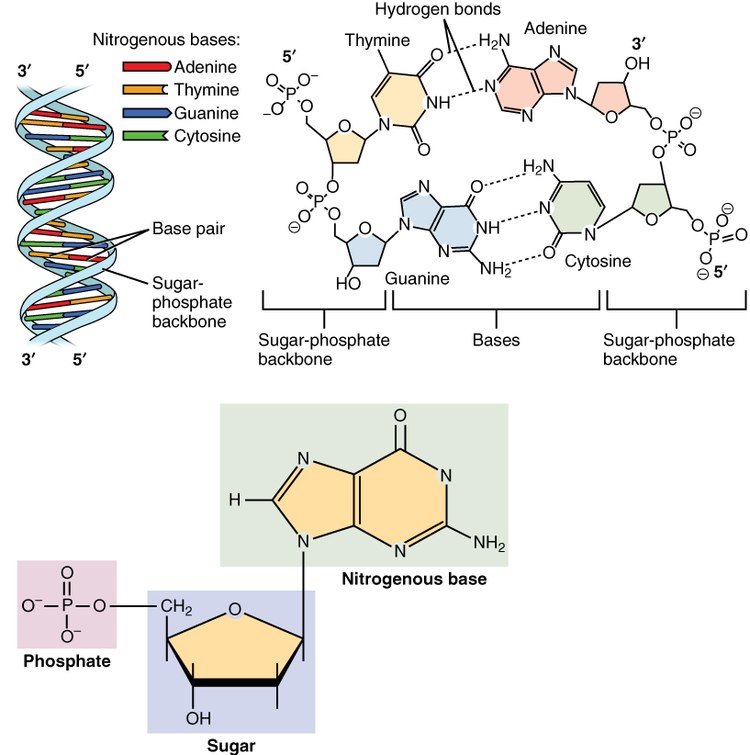
green background (right top) nitrogen base.
blue background (low center) pentosa sugar (in the form of pentagon).
pink background (low left) phosphate.
The nucleotides are small molecules synthesized by all living organisms, which are formed by the union of three elements: a nitrogenous base, a simple sugar and a phosphate group. Each nucleotide is an assembly (by covalent bonding) of: a pentose, a nitrogenous base, one or more phosphate groups. The part of the nucleotide formed only by the nitrogenous base and the pentose is called the nucleoside.
- Nitrogenated bases: derived from the purine and pirimidine heterocyclic compounds.
- Purin nitrogenated bases: adenine (A) and guanine (G). Both are part of DNA and RNA.
- Pyrimidinic nitrogen bases: are thymine (T), cytosine (C) and uracyl (U). Timin and cytosine are involved in DNA formation. In the RNA, cytosin and uracyl appear.
- Isoaloxacynic nitrogen bases: flavina (F). It is not part of DNA or RNA, but it is from some important compounds such as FAD.
- Pentosa: the sugar of five carbon atoms; it can be ribose (RNA) or desoxyribose (DN). The difference between both is that RNA does have an OH group in the second carbon.
- Phosphoric acid: H formula3PO4. Each nucleotide may contain one (nucleotides-monophosphate, such as AMP), two (nucleotides-diphosphate, such as ADP) or three (nucleotides-triphosphate, such as ATP) phosphate groups.
Nomenclature
The position of atoms in a nucleotide is numbered relative to the carbon atoms in the ribose or deoxyribose sugar.
- Purine or pirimidine is located in carbon 1 of sugar.
- The phosphate group is in carbon 5.
- The hydroxyl group is linked to carbon 3 of sugar. It can be released in the form of water from the formation of the fosfodiester link.
- There may be an additional hydroxyl group linked to carbon 2, if the pentase is a ribose and a carbonyl.
Summary
Nucleotide synthesis can occur de novo or recovery. The de novo pathway uses phosphoribosyl pyrophosphate (PRPP), to which simple molecules (CO2, amino acids and tetrahydrofolate) are added, finally composing the purine and pyrimidine nucleotides.
Functions of nucleotides
Nucleotides are fundamental biomolecules that carry the genetic information for cell replication. Nucleotides also play several essential roles in energy transfer, and regulate many metabolic pathways.
Genetic Information
The sequence of nitrogenous bases that make up each nucleotide is what creates the genetic code of the information contained in DNA. Nucleotides are arranged in two long chains that form a spiral called a "double helix". Protein-coding genes are made up of tri-nucleotide units called codons.
Energy transfer
Nucleotides are molecules with a lot of energy accumulated in the bonds of the phosphate groups, which is why they are used in all types of cells for the transfer of energy in metabolic processes.
Nucleotides are in a stable state when they have only one phosphate group. Each additional phosphate group possessed by a nucleotide is in a more unstable state, and the phosphorus-phosphate bond tends, when broken by hydrolysis, to release the energy that binds it to the nucleotide.
Cells have enzymes whose function is precisely to hydrolyze nucleotides to extract the energy potential stored in their links. For this reason, a triphosphate nucleotide is the most used source of energy in the cell. Of these, ATP (an adenine nucleotide with three energy-rich phosphate groups) is the central axis in cellular reactions for the transfer of the energy demanded. UTP (uracil + three phosphates) and GTP (guanine and three phosphates) also meet the energy demands of the cell in reactions with sugars and changes in protein structures, respectively.
Degradation of nucleotides
Nucleotides are made of purine and pyrimidine and are continually broken down and/or recycled in most living organisms.
During digestion, nucleic acids are hydrolyzed to oligonucleotides by enzymes called nucleases, continuously, oligonucleotides are hydrolyzed by various phosphodiesterases in a process that produces a mixture of mononucleotides.
The nucleotidases remove the phosphate groups from nucleotides, producing nucleosides. These latter molecules are hydrolyzed by nucleosidases to free bases and ribose or deoxyribose, which are then absorbed.
The dietary bases of purine and pyrimidine are not used in amounts to synthesize cellular nucleic acids. Instead, they are broken down within enterocytes. Purines are broken down to uric acid in humans and birds. Pyrimidines degrade to Berta alanine or Berta aminoisobutyric acid, as well as NH3 and CO2. Unlike catabolic processes for other major classes of biomolecules (eg, sugars, fatty acids, and amino acids), purine and pyrimidine catabolism does not result in ATP synthesis.
The main pathways for the degradation of purine and pyrimidine bases:
Contenido relacionado
Natural Sciences
Posidoniaceae
Calathea