Nucleic acid
Nucleic acids are large polymers formed by repeating monomers called nucleotides, linked by phosphodiester bonds. Long chains are formed; some nucleic acid molecules reach gigantic sizes, of millions of chained nucleotides. There are two basic types, DNA and RNA.
The discovery of nucleic acids is due to Johan Friedrich Miescher who, in 1868, isolated from the nuclei of cells an acidic substance which he called nuclein, a name that later became changed to nucleic acid. Later, in 1953, James Watson and Francis Crick discovered the structure of DNA from Photograph 51, made by Rosalind Franklin using the X-ray diffraction technique.
Importance of Nucleic Acids
Nucleic acids are biomolecules, in the same way as carbohydrates, lipids and proteins, and for this reason, all living beings possess them. Nucleic acids are the molecules that contain genetic information, information that directs and controls the synthesis of proteins in an organism. Nucleic acids provide the information that determines the specificity and biological characteristics of proteins. And proteins are polymers made up of monomers called amino acids and linked by peptide bonds, which determine the shape and structure of cells and direct almost all vital processes.nucleic acid" It is used to describe specific, large molecules in the cell. They are actually made of repeating chains of polymer units; the two most famous nucleic acids you may have heard of are DNA and RNA
Types of nucleic acids
There are two types of nucleic acids: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), which are differentiated:
- For the glucid (the pentosa is different in each; ribose in the RNA and deoxyribose in the DNA);
- By the nitrogen bases: adenine, guanine, cytosine and thymine, in DNA; adenine, guanine, cytosine and uracyl, in RNA.
- By the number of strings: while DNA is a bicatenary molecule that forms a double helix, RNA has only one string, that is, monocatenary.
Nitrogenous bases
Nitrogenous bases are those that contain genetic information, they present a cyclic structure that contains carbon, nitrogen, hydrogen and oxygen. They are divided into two types:
- Purins, which are derived from purine (two rings).
- Pirimidins, derived from the ring of the pirimidine (a ring).
The presence of nitrogen atoms gives these compounds a basic character. They are aromatic and therefore they are flat, they are also insoluble in water and can establish hydrophobic interactions between them; these interactions serve to stabilize the three-dimensional structure of nucleic acids. The existence of different radicals means that several nitrogenous bases can appear, which are:
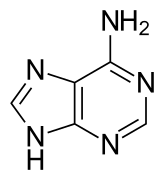
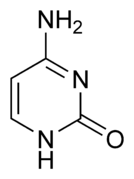
- Adenine, present in DNA and RNA
- Guanina, present in DNA and RNA
- Citosin, present in DNA and RNA
- Timina, present exclusively in DNA
- Uracilo, present exclusively in RNA
Nucleosides and nucleotides
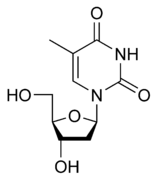
A nucleoside is a unit made up of a pentose (ribose or deoxyribose) attached to a nitrogenous base. The union is made through an N-glycosidic bond, with a beta (β) configuration, which is a variant of the glycosidic bond, which is formed when an intramolecular hemiketal reacts with an amine, instead of with an alcohol, releasing a molecule of water. In nucleosides it is carried out between carbon 1 (carbonyl) of the sugar and one of the nitrogen atoms of the nitrogenous base, if this is a pyrimidine it is attached to the 1' and if it is a purine in position 9'.
The planes of the base and the sugar are perpendicular to each other but the bases can present two different conformations:
- "anti" when the plane of the base is removed from the plane of the pentosa.
- "syn" when the bases are on the pentase plane.
There are two types of nucleosides:
- Ribonucléics containing β-D-ribosa.
- Desoxirribonucléics containing β-D-desoxirribosa.
To name these compounds, one must take into account what nitrogenous base it is and what sugar it is attached to; when it is a purine base, the ending "-osine" is added to its name and the ending "-idine" if it is a pyrimidine and the prefix "deoxy-" is added in the case of deoxyribonucleosides.
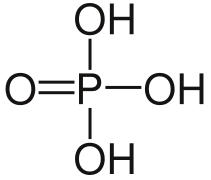
The nucleotides are the basic units of nucleic acids and chemically they are the phosphoric esters of nucleosides, that is, they are the result of the union between a ribose, a nitrogenous base and a phosphoric acid. The union between the nucleoside and the phosphoric acid is carried out by means of an ester bond that can occur in any of the free hydroxyl groups of the pentose, but as a general rule it takes place in the alcohol group of the 5' carbon. Nucleotides can contain from one to three phosphate groups, linked one after the other, for example monophosphate containing only one phosphate group, diphosphate with two, triphosphate with three. The presence of the phosphate group, which is ionized at pH 7, gives the molecule a markedly acidic character.
Like nucleosides, nucleotides are also divided into two groups depending on the ribose they contain:
- Ribonucleotides if they have ribs.
- Desoxyribonucleotides if they have deoxyribose.
There are different ways to name these compounds, the most used and the simplest way is where each nucleotide is identified with three capital letters. The first one corresponds to the nitrogenous base that contains the nucleotide, the second letter indicates whether it is a mono-, di- or triphosphate and the third is the initial of the phosphate group, which is a P and finally, in the case of deoxyribonucleotides, a lowercase d is placed before the three letters. Another way of naming them is to put the word acid at the beginning and then the name of the nitrogenous base is placed with the ending -yl, but this naming system can be a bit ambiguous since the number of phosphate groups cannot be known. containing the nucleotide. They are also usually named as the phosphates of the corresponding nucleosides.
For example: You want to name the nucleotide composed of an adenine with a phosphate group and a ribose.
- To use the three-letter method, the nitrogenated base is first identified, which is an Adenine and therefore the first letter is an A, the second letter corresponds to the number of phosphate groups which is only one and therefore the second letter is an M of monophosphate, and finally the letter P. The name of the nucleotide would be AMP. In case instead of being a ribose it was a deoxyribose the letter d was placed at the beginning, dAMP.
- With the second form the word acid and adenine is placed as adenylic, therefore the name of the nucleotide would be adenylic acid.
- Finally the name of the corresponding nucleotide is required, which is adenosine and phosphate is added, and the full name would be adenosine phosphate.
In addition to forming the structure of nucleic acids, nucleotides have other relevant functions:
- Adenosine nucleoside has neurotransmitter functions.
- ATP is the universal molecule for energy transfer.
- UDP and CDP serve as transporters in the metabolism of glucids, lipids and other molecules.
- AMPc, GMPc and ATP itself perform regulatory functions.
- AMP is part of the coenzyme structure such as FAD, NAD+, NADP+ and CoA.
Characteristics of DNA
DNA is double-stranded, it is made up of two polynucleotide chains joined together for their entire length. This double chain can be arranged linearly (DNA from the nucleus of eukaryotic cells) or circularly (DNA from prokaryotic cells)., as well as eukaryotic mitochondria and chloroplasts). The DNA molecule carries the necessary information for the development of the biological characteristics of an individual and contains the messages and instructions for the cells to carry out their functions. Depending on the composition of the DNA (referring to composition as the particular sequence of bases), it can denature or break the hydrogen bonds between bases becoming single-stranded DNA or scDNA for short.
Unusually, the DNA of some viruses is single-stranded.
DNA is a relatively stable polymer. Spontaneous reactions, such as deanimation of certain bases, hydrolysis of base-sugar N-glycosidic bonds, radiation-induced formation of pyrimidine dimers, occur slowly but are important because the cell has a low tolerance to changes in the genetic material.
DNA sequence can be determined and DNA polymers can be synthesized by a regulation that incorporates chemical and enzymatic methods.
DNA Structures
- Primary structure. A chain of deoxyribonucleotides (monocatenary) that is, is formed by a single polynucleotide, without a complementary chain. It's not functional, except in some viruses.
- Secondary structure. Double helix, bicatenary structure, two complementary nucleotide chains, anti-parallel, joined together by the nitrogenated bases through hydrogen bridges. It is helically rolled around an imaginary axis. There are three types:
- Double helix A, with dextrogy twist, but turns are in a tilted plane (non-coding DNA).
- Double helix B, with dextrogy twist, perpendicular turns (functional DNA).
- Double helix Z, with levour spin, perpendicular turns (not functional); it is present in the parvovirus.
RNA Characteristics
RNA differs from DNA in that the pentose of the constituent nucleotides is ribose instead of deoxyribose, and that instead of the four bases A, G, C, T, A, G, C, U (that is, uracil instead of thymine). RNA chains are shorter than DNA chains, although this characteristic is due to biological considerations, since there is no chemical limitation to form RNA chains as long as DNA, since the phosphodiester bond is chemically identical. RNA is almost always made up of a single strand (it is single-stranded), although in certain situations, such as tRNA and rRNA, it can form complex and stable folded structures.
While DNA contains information, RNA expresses that information, going from a linear sequence of nucleotides to a linear sequence of amino acids in a protein. To express such information, several steps are needed and, consequently, there are several types of RNA:
- The RNA messenger it is synthesized in the core of the cell, and its base sequence is complementary to a fragment of one of the DNA chains. It acts as an intermediary in the transfer of genetic information from the core to the cytoplasm. Shortly after its synthesis it comes out of the core through the nuclear pores associating itself to the ribosomes where it acts as a matrix or mould that orders the amino acids in the protein chain. His life is very short: once his mission is accomplished, it is destroyed.
- The Transfer RNA It exists in the form of relatively small molecules. The only strand of which the molecule consists can come to present areas of secondary structure thanks to the hydrogen bridge links that form between complementary bases, which leads to the formation of a series of arms, loops or handles. Its function is to capture amino acids in the cytoplasm by joining them and transporting them to the ribosomes, placing them in the right place that indicates the sequence of nucleotides of the messenger RNA to reach the synthesis of a determined polypeptide chain and therefore to the synthesis of a protein
- The Ribonomic RNA is the most abundant (80 percent of total RNA), it is found on the ribosomes and is part of them, although there are also ribosome proteins. The newly synthesized ribosome RNA is immediately packaged with ribosome proteins, leading to the subunits of the ribosome.
Chemistry of Nucleic Acids
DNA and RNA can be denatured.
Elevation of temperature and extreme pH values cause denaturation of double-helical DNA (generally it happens at its melting point temperature). This causes the unwinding of the double helix, due to the destabilization of the hydrogen bonds between the base pairs, there is no breaking of covalent bonds.
Renaturation is a rapid process that consists of a single step, for this there must be a double helix segment of a dozen or more residues that hold the two strands together. When the pH and temperature return to normal values, what was uncoiled spontaneously coils up again. But if the two strands are completely separated, it is carried out in two steps. In the first, the process is slow, the DNA strands recognize each other randomly and form a small double helix fragment. In the second, the process is faster and the unpaired bases progressively pair to form the double helix.
Hypochromic effect.
When close interactions of the stacking of the nucleic acid bases occur, they produce a decrease in the absorption of UV light, in relation to the absorption of a solution of free nucleotides of the same concentration; adsorption decreases when the double chain is formed. This phenomenon is known as the hypochromic effect.
When a nucleic acid is denatured, an opposite effect is produced, there is an increase in adsorption, it is called hyperchromic.
The DNA molecules of a virus or a bacterium in solution are denatured at their melting point (the tm; melting temperature or melting temperature of the DNA is the temperature at which 50% of the DNA has its strands separated). The higher the content of cytosine and guanine bases (C≡G), the higher the melting point, since these two bases are linked by three hydrogen bonds, unlike adenine and thymine which are linked by two.
Artificial nucleic or ribonucleic acids
There are, apart from the natural ones, some nucleic acids not present in nature (nucleic acid analogues), synthesized in the laboratory.
- Peptide nucleic acid, where the phosphate-(desoxi)ribosa skeleton has been replaced by 2-(N-aminoetil)glicin, coupled with a classic peptide link. Puric and pyramidal bases join the skeleton by carbon dioxide. In the absence of a charged skeleton (the phosphate ion carries a negative charge to physiological pH in the DNA/ARN), it is more strongly joined to a complementary chain of monocatenary DNA, as there is no electrostatic repulsion. The force of interaction grows when a bicatenary ANP is formed. This nucleic acid, not being recognized by some enzymes due to its different structure, resists the action of nucleases and proteases.
- Morfolin and blocked nucleic acid (LNAin English. Morfolin is a derivative of a natural nucleic acid, with the difference that it uses a morphine ring instead of sugar, conserving the fosfodiéster link and the nitrogenated base of natural nucleic acids. They are used for research purposes, usually in the form of 25 nucleotides. They are used to make reverse genetics, as they are able to complement pre-ARNm, thus avoiding their subsequent cut and processing. They also have a pharmacist use, and may act against bacteria and viruses or to treat genetic diseases by preventing the translation of a certain RNA.
- Glycolic nucleic acid. It is an artificial nucleic acid where the ribose is replaced by glicerol, keeping the base and the fosfodiéster link. It does not exist in nature. It can be complementary to DNA and RNA, and surprisingly makes it more stable. It is the chemically simplest form of a nucleic acid and is speculated that it has been the ancestral precursor of the current nucleic acids.
- Treosic nucleic acid. It differs from natural nucleic acids in skeleton sugar, which in this case is a treasury. ATN-ADN hybrid chains have been synthesized using polymerase DNA. It is complementary to RNA, and may have been its precursor.
- Chimeroplast. It is a molecule formed by the mixture of DNA and RNA that is used in gene therapy.
Contenido relacionado
Deinonychus antirrhopus
Hydrochoerus hydrochaeris
Tenantism