Nuclear processes
The pro-nuclear processes are processes of combination and partial elaboration of subatomic particles and atomic nuclei. Nuclear reactions can be endothermic or exothermic, depending on whether they atomize energy to produce it or release it respectively.
Forces
More information in: Fundamental forces
- Nuclear strong: It is the strongest force of nature and has, in principle, very short reach, 1 fm. She's responsible for nuclear ligatures. Among hadrons is manifested through the exchange of mesones and piones above all. But the true expression of strong nuclear occurs in the connections between quarks through a mediating force particle called gluon that comes from "glue" which means glue. The gluons unite with such firmness to the quarks that so far they have not been observed free in nature but always appear bound to, at least, another quark. Here the force acts with infinite reach and increases with distance that is to say the further we move two quarks the more strongly they attract. Since it is a force derived from the attractions between quarks those particles that are not constituted by these as the leptons are not affected by it.
- Nuclear weak: It is the least powerful force, 1 am distance is less than the core, it is also 1013 Sometimes weaker than strong nuclear. Its force mediating particles are the W and Z Bones. It is responsible for most radioactive processes.
- Electromagnetic: This is a powerful force, in reality infinite reach. It is also a very strong force only a hundred times weaker than strong nuclear power.
- Gravitational: weak and far-reaching. Act on mass and energy. She's always attractive. Totally despicable in nuclear reactions as it is 10^38 times weaker than strong nuclear.
Types of particles
More information at: Standard Model | Particle table | Particle List
According to its spin:
- Bonuses: entire spine particles (0, 1, 2...). It's photons and messones.
- Fermiones: Semi-entero Spine particles (1/2, 3/2...). It's the bars, the quarks and the leptons.
Depending on their structure or interactions in which they may be involved:
- Hadrons: particles formed by quarks. It's the mesons and the bars.
- Months: Hadrones formed by two quarks.
- Bariones: Hadrones formed by three quarks. It's protons and neutrons.
- Leptons: Fundamental particle in indivisible principle that does not experience strong interaction. It's electrons, muons, tau and neutrinos.
- Quarks: Basic particle in in indivisible principle that must necessarily appear linked to other quarks to form hadrons. The protons and neutrons are composed of three quarks. It has a fractional electric charge and also interacts weakly (if the quark is left). Experience strong interaction.
- Antiparticles: Each particle has its own associated antiparticle. These have equal mass but opposite load. Or, when they have no electric charge, they have another characteristic opposite property like helicity in the case of neutrino and antineutrino, if they had no mass. In any case, it would be possible for the neutrino and the antineutrino to be Majorana's same fermionic particle if, finally, although small, it is measured that they have no null mass.
Conservation laws
Every nuclear process must comply with a formalism similar to that followed by chemists in chemical reactions. In fact, in terms of symbology, both types of processes are written in a fairly similar way. If in chemical reactions the atomic mass was conserved in nuclear ones, the same thing no longer happens. Since there are transformations of mass to energy and vice versa. Despite this, nuclear processes follow their own conservation laws.
- Relativistic energy: Relativistic energy is the sum of the kinetic energies of particles and their resting energies. This is preserved during any nuclear reaction.
- Electric charge: The total value of electrical loads on both sides of the equation must be maintained. The load unit is that of the electron and is represented by qe.
- Barionic Number: The value +1 is assigned to the bars and -1 to the antibarions. The value during the reaction must remain constant.
- Leptonic Number: The value +1 is assigned to the lepttons and -1 to the antilepttons. The value during the reaction must remain constant. There is a leptonic number for each generation of particles so in fact there are three, an electronic leptonic number, another muonic and another tauonic and in fact all of them are to be kept separately.
- Strangeness: The value 0 is assigned to normal particles, photons, leptons or pions and +1 or -1 to foreign particles and antiparticles such as k or kaons. These have a half-life above normal and come up by pairs. This value is preserved during reaction only in strong electromagnetic or nuclear interactions, not in the weak.
Note: Probably, without the conservation of baryon and lepton numbers and strangeness, the universe today would just be a soup of leptons or even smaller particles that would have degraded from one to another. irreversible form.
Energy per nucleon
It is the nuclear potential energy contained in each nucleon of an atom. This energy varies according to the atom. This fact is what is normally known as a mass defect and is the cause of the fission and fusion reactions releasing energy. To give an example, this curious phenomenon causes an isolated neutron and a proton to add more mass than both together forming a deuterium nucleus. In the attached image there is a graph in which you can see some of these energies.
An approximate way to obtain that potential energy is to calculate the rest energy of an atomic nucleus from its atomic mass. Immediately afterwards, that energy must be divided by the number of nucleons in that nucleus. Then that value must be subtracted from the rest energy of hydrogen, about 938MeV. To see the atomic masses of each isotope: webelements. More detailed calculations in: Mass defect
The function in the figure has a maximum, the iron peak. Iron is the most nuclear stable element of all because both to fuse it and to fission it is necessary to invest additional energy. The reasons that explain the shape of this graph are as follows. For light atoms the strong nuclear force is dominant but this force only acts at a very short range while the electromagnetic repulsive forces between protons are long-range and always act on all protons. In the heavier nuclei, however, the distances between many of the nucleons are too great and the cohesion by strong interaction is no longer as strong. On the other hand, the electromagnetic repulsion forces are getting stronger since there are more protons and these are long-range. Thus, starting with iron, the electric potential barrier that must be broken to add one more proton to the nucleus exceeds the energetic benefit that the strong interaction gives when joining it with the rest of the nucleons. This also explains the gentle slope of the energy obtained by fission since it is really given by the excess electrical potential above the cohesion by strong interaction while the fusion energy is the opposite, the energy is provided by the strong interaction. which far exceeds the repulsive forces, especially in the lightest atoms such as hydrogen or helium with hardly any positive charges.
Radioactive decay
It occurs when an unstable nucleus or particle spontaneously decays into another nucleus or particle, emitting some type of radiation in the process. For example the beta decay of a neutron:
n→ → p+e− − +.. ! ! e{displaystyle nrightarrow p+e^{-}+{bar {nu }}_{e}}Barionic: 1 = 1 (contained)
· Leptonic: 0 = 1 -1 (preserved)
Photodisintegration
It happens in a similar way to spontaneous decay, only this time the process is induced by an external gamma photon. This reaction is endothermic.
- Examples:
Barionic: 20 = 16 + 4
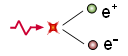
Creation and annihilation of pairs
Although a photon in a vacuum cannot create pairs of particles, it can if it is energetic enough and interacts with another photon or another particle. The generated pair can be electron/positron or proton/antiproton, for example. The type of particle generated will depend on the frequency or energy of the photon.
γ γ ▪ ▪ e− − +e+{displaystyle gamma leftrightarrow e^{-}+e^{+}}Number of leptonics: 0 = 1 - 1
For the electron/positron pair, for example, the gamma photon will have to have an energy greater than 1022keV since each electron has 511keV of energy at rest and a certain kinetic energy is always needed for them to separate from each other.
Likewise, these pairs can later annihilate each other by generating two or more high-frequency gamma photons.
Neutron capture
As explained above, nuclear fusion can only reach the peak of iron. To create nuclei heavier than this element, different types of reactions are needed. Neutron capture is a simple reaction. Neutrons, being neutral particles, do not have to overcome an electromagnetic potential barrier, so they can easily collide with any nucleus. Doing so results in an isotope with a higher mass number but the same atomic number since the number of protons does not change. This process can be repeated until the neutron-charged nucleus becomes so unstable that beta decay occurs faster than another neutron is absorbed. When this happens, the nucleus increases its atomic number but keeps its mass intact. Doing so increases its stability and can continue to capture neutrons. And so, little by little, the atoms fatten up to elements heavier than iron.
The attached diagram represents the number of protons (Z) as a function of the number of neutrons (N). N grows until the characteristic time of beta decay is less than that of neutron capture, at which time a transmutation of the nucleus occurs, becoming a different element. The amount of neutrons that it manages to add before being transmuted will depend on the intensity of the neutron flux to which the initial nucleus is subjected.
Depending on whether the flow is fast (rapid) or slow (slow), it will be called process-r or process-s respectively. There are atoms that can be produced by only one of the processes and others that can be produced by both.
These intense flows of neutrons occur naturally in supernovae, which is where most of the elements heavier than iron are synthesized. Even so, there are atoms that cannot be produced by this process.
Proton Capture
This process is also relatively likely. Although a proton has some electrical charge, it doesn't have much of an electrical charge either, and it doesn't take a lot of energy to break the potential barrier. Proton capture increases the atomic number and the mass number at the same time.
Electron Capture
It is the process by which electrons are captured by nuclei, thus transforming protons into neutrons. That is why the process is also called neutronization. It occurs, above all, during the formation of neutron stars.
p++e− − → → n+.. e{displaystyle p^{+}+e^{-}rightarrow n+nu _{e}
| e− − +7Be→ → 7Li+.. e− − {displaystyle e^{-}+^{7}mathrm {Be} rightarrow ^{7}mathrm {Li} +nu _{e}^{-}}}
|
Contenido relacionado
Specific impulse
Electronvolt
Simeon Denis Poisson