Nuclear fusion
In nuclear physics, nuclear fusion is a nuclear reaction in which several atomic nuclei unite to form a heavier nucleus.These reactions are, in general, exothermic when they occur between larger atoms. lighter than iron and endothermic if heavier. This energy absorbed or released during the reaction is due to the effect known as mass defect. Fusion reactions, mediated by strong nuclear interaction, occur when the light atoms to be fused have enough energy to overcome the repelling electromagnetic forces. These conditions only occur at high temperatures, when the matter that these atoms form is in a plasma state. Nuclear fusion is, therefore, the reverse process of nuclear fission, a nuclear reaction in which a heavy atom gives rise to two lighter atoms.
Given the necessary temperature, in nature this type of reaction can only be observed in the core of stars, where temperatures are on the order of tens of millions of Kelvin. The energy released in the fusion reactions that occur in stars is, in fact, their main source of energy. In addition, these fusion reactions that occur in stars are one of the main sources of the creation of naturally occurring heavy chemical elements, in a process known as stellar nucleosynthesis.
Fusion reactions can also be carried out artificially, although due to the necessary temperature conditions, controlling these reactions is an unresolved technological challenge. The first practical application of nuclear fusion was the invention of the hydrogen bomb. At present, research is also being done on how to maintain the necessary conditions for nuclear fusion reactions to occur in a controlled manner and thus be able to generate electrical energy from it. In particular, since the energy released during the reaction is greater the smaller the mass of the intervening atoms, fusion reactions that occur between different isotopes of hydrogen are often investigated. For the control of the reaction there are different concepts in development, mainly inertial confinement and magnetic confinement. This research is carried out in different laboratories and organizations around the world, the most notable being the ITER project, the result of international collaboration and focused on magnetic confinement.
History
On the basis of Ernest Rutherford's nuclear transmutation experiments, conducted a few years earlier, Mark Oliphant, in 1932, first observed the fusion of light nuclei (hydrogen isotopes). Later, for the rest of that decade, Hans Bethe studied the stages of the main cycle of nuclear fusion in stars. Research into military fusion began in the 1940s as part of the Manhattan Project, but was unsuccessful until 1952. Research into controlled fusion for civilian purposes began in the 1950s and continues to the present day. Present.
On December 5, 2022, the NIF (National Ignition Facility) achieved, in a nuclear fusion experiment by inertial confinement, a positive gain factor, that is, it achieved that the nuclear fusion reaction carried out released more energy than put into the fuel.
Physics of the process
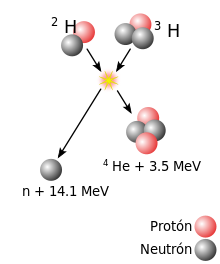
For fusion to occur, an important energy barrier produced by the electrostatic force must be overcome. At great distances, two nuclei repel each other due to the electrostatic repulsive force between their positively charged protons. However, if two nuclei can be brought close enough, due to the strong nuclear interaction, which is greater at short distances, the electrostatic repulsion can be overcome.
When a nucleon (proton or neutron) is added to a nucleus, the nuclear force attracts other nucleons, but—due to the short range of this force—mainly its immediate neighbors. Nucleons inside a nucleus have more neighboring nucleons than those on the surface. Since the surface area to volume ratio of smaller nuclei is larger, the binding energy per nucleon due to the nuclear force generally increases with the size of the nucleus, but approaches a limiting value corresponding to that of a nucleus. whose diameter is equivalent to that of almost four nucleons. On the other hand, the electrostatic force is inverse to the square of the distance. Thus, a proton added to a nucleus will be affected by an electrostatic repulsion of all other protons. Therefore, due to the electrostatic force, as the nuclei get larger, the electrostatic energy per nucleon increases without limit.
The net result of these opposing forces is that the binding energy per nucleon generally increases with the size of the nucleus, until it reaches the elements iron and nickel, and a further decrease in heavier nuclei. Eventually the nuclear binding energy becomes negative, and the heavier nuclei (with more than 208 nucleons, corresponding to a diameter of about six nucleons) are not stable. Four very tightly bound nuclei, in order of decreasing nuclear binding energy, are 62Ni, 58Fe, 56Fe, and 60Ni. Although the nickel isotope 62Ni is more stable, the iron isotope 56Fe is an order of magnitude more stable. common. This is due to the higher decay rate of 62Ni inside stars, driven by photon absorption.
A notable exception to this general trend is the nucleus helium 4He, whose binding energy is greater than that of lithium, the next element by weight gain. An explanation for this exception is provided in the Pauli exclusion principle: because protons and neutrons are fermions, they cannot exist in the same state. Because the nucleus of 4He is composed of two protons and two neutrons, so that its four nucleons can be in the ground state, its binding energy is abnormally large. Any additional nucleons would have to be located in higher energy states.
Three advantages of nuclear fusion are:
a) to a large extent its waste does not have the problems of those from fission;
b) abundance –and good price–[citation needed] of raw materials, mainly the hydrogen isotope deuterium (D);
c) if a facility were to stop working, it would shut down immediately, with no danger of non-nuclear fusion.
In a promising design, to start the reaction, several high-powered laser beams transfer energy to a small fuel pellet, which heats up and implodes: from all points it collapses and is compressed to a minimum volume, which causes nuclear fusion.
Plasma confinement
Magnetic confinement
Inertial Confinement
Stable electrostatic confinement for nuclear fusion
As you can see in the drawing above, it is based on total circumscription of electrostatically confined hydrogen ions. The benefits of this confinement are multiple:
- The thickness of the copper sphere overrides the instability caused by symmetry errors.
- Hydrogen ionization is easily generated by the electric field that absorbs electrons without diminishing the intensity of that field.
- An intense electric field can be obtained, which would prevent hydrogen ions from leaking.
- The necessary energy is less than that consumed by a fusion reactor that generates an electromagnetic field to confine ions.
Nuclear fusion is achieved by means of compression-decompression, increasing or decreasing the intensity of the electric field. To do this, the speed of the electricity generator is increased or decreased. Lead can be used as a neutron moderator, although its effectiveness would have to be proven.
Other media
- Pinzamiento
- Gravitational confidence
Contenido relacionado
Gravitational singularity
Sun
Barycenter