Nuclear fision
In nuclear physics, fission is the splitting of a nucleus into lighter nuclei, plus some byproducts like free neutrons, photons (usually gamma rays), and other fragments of the nucleus like alpha particles (helium nuclei) and beta (high-energy electrons and positrons) as well as a large amount of energy. Its discovery is due to Otto Hahn and Lise Meitner, although he was the first to receive the Nobel Prize for it.
Nuclear fission of heavy elements was discovered on December 17, 1938 by the German Otto Hahn and his assistant Fritz Strassmann at the suggestion of the Austro-Swedish physicist Lise Meitner who explained it theoretically in January 1939 together with her nephew Otto Robert Frisch. Frisch named the process by analogy with the binary fission of living cells. In the case of heavy nuclides, this is an exothermic reaction that can release large amounts of energy, both in the form of electromagnetic radiation and kinetic energy of the fragments. Like nuclear fusion, for fission to produce energy, the total binding energy of the resulting elements must be greater than that of the starting element.
Fission is a form of nuclear transmutation because the resulting fragments (or daughter atoms) are not the same element as the original parent atom. The two (or more) nuclei produced are usually of comparable but slightly different sizes, typically with a mass ratio of the products of about 3 to 2, for common fissiles, common isotope. Most fissions are binary (produce two charged fragments), but occasionally (2-4 times per 1000 events), three positively charged fragments are produced in ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.
Aside from neutron-induced fission, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also called fission, and it occurs especially at very high number isotopes. dough. Spontaneous fission was discovered in 1940 by Fliorov, Pétrzhak, and Kurchatov in Moscow, in an experiment that sought to confirm that, without neutron bombardment, the uranium fission rate was negligible, as Niels Bohr had predicted; it was not negligible.
The unpredictable composition of the products (which vary in a largely probabilistic and somewhat chaotic manner) distinguishes fission from pure tunneling processes such as proton emission, alpha decay, and cluster decay, which yield the same products every time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when broken. This makes possible a self-sustaining nuclear chain reaction, releasing energy at a controlled rate in a nuclear reactor or at a very fast and uncontrolled rate in a nuclear weapon.
The amount of free energy contained in nuclear fuel is millions of times greater than the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. However, the products of nuclear fission are, on average, much more radioactive than the heavy elements that are normally fissioned as fuel, and remain so for a long time, giving rise to a nuclear waste problem. Concern about the accumulation of nuclear waste and the destructive potential of nuclear weapons is in contrast to the peaceful desire to use fission as a source of energy.
Mechanism
Fission of heavy nuclei is an exothermic process, which means that substantial amounts of energy are released. The process generates much more energy than that released in conventional chemical reactions, in which electronic shells are involved; energy is emitted, both in the form of gamma radiation and the kinetic energy of the fission fragments, which will heat the matter around the space where the fission occurs.
Fission can be induced by several methods, including bombarding the nucleus of a fissile atom with a particle of the correct energy; the particle is generally a free neutron. This free neutron is absorbed by the nucleus, making it unstable. The unstable nucleus will then split into two or more pieces: the fission products include two smaller nuclei, up to seven free neutrons (averaging two and a half per reaction), and some photons.
Atomic nuclei released as fission products can be various chemical elements. The elements that are produced are the result of chance, but statistically the most likely result is to find nuclei with half the protons and neutrons of the original fissioned atom.
Fission products are generally highly radioactive, not stable isotopes; these isotopes then decay, via decay chains.
Radioactive decay
Nuclear fission can occur without neutron bombardment as a type of radioactive decay. This type of fission (called spontaneous fission) is rare, except for a few heavy isotopes.
Nuclear Reaction
In engineered nuclear devices, essentially all nuclear fission occurs as a 'nuclear reaction,' a bombardment-driven process that results from the collision of two subatomic particles. In nuclear reactions, a subatomic particle collides with an atomic nucleus and causes changes in it. Nuclear reactions are therefore driven by the mechanics of the bombardment, and not by the relatively constant exponential decay and half-life characteristic of spontaneous radioactive processes.
Many types of nuclear reactions are currently known. Nuclear fission differs significantly from other types of nuclear reactions in that it can be amplified and sometimes controlled by a nuclear chain reaction (a type of general chain reaction). In such a reaction, the free neutrons released by each fission event can trigger still more events, which in turn release more neutrons and cause more fissions.
Chemical isotopes that can sustain a chain fission reaction are called nuclear fuel and are said to be fissile. The most common nuclear fuels are 235U (the uranium isotope with mass number 235 and used in nuclear reactors) and 239Pu (the plutonium isotope with mass number 239). These fuels break down into a bimodal range of chemical elements with atomic masses centered near 95 and 135 u (fission products). Most nuclear fuels undergo spontaneous fission only very slowly, instead decaying primarily through alpha-beta over periods of millennia to eons. In a nuclear reactor or nuclear weapon, the vast majority of fission events are induced by bombardment with another particle, a neutron, which in turn is produced by earlier fission events.
Nuclear fission in fissile fuels is the result of the nuclear excitation energy produced when a neutron is captured by a fissile nucleus. This energy, resulting from the capture of the neutron, is the result of the attractive nuclear force acting between the neutron and the nucleus. It is enough to deform the core into a "drop" double-lobed, to the point that the nuclear fragments exceed the distances that the nuclear force can hold together two groups of charged nucleons, and when this occurs, the two fragments complete their separation and are then further separated by their charges mutually repulsive, in a process that becomes irreversible with increasing distance. A similar process occurs in fissile isotopes (such as uranium-238), but in order to fission, these isotopes require additional energy provided by fast neutrons (such as those produced by nuclear fusion in thermonuclear weapons).
The liquid droplet model of the atomic nucleus predicts fission products of equal size as a result of nuclear deformation. The more sophisticated nuclear shell model is needed to mechanistically explain the path to the most energetically favorable outcome, in which one fission product is slightly smaller than the other. A theory of fission based on the shell model has been formulated by Maria Goeppert Mayer.
The most common fission process is binary fission, and it produces the fission products noted above, at 95±15 and 135±15 u. However, the binary process occurs simply because it is the most likely. At anywhere from 2 to 4 fissions per 1000 in a nuclear reactor, a process called ternary fission produces three positively charged fragments (plus neutrons) and the smallest of these can range from as small a charge and mass as a proton (Z = 1), up to a fragment as large as argon (Z = 18). However, the most common small fragments are 90% composed of helium-4 nuclei with more energy than the alpha particles of alpha decay (so-called "long-range alphas" at ~16 MeV), as well as helium-6 nuclei, and tritons (the tritium nuclei). The ternary process is less common, but still produces a significant accumulation of helium-4 and tritium gas in the fuel rods of modern nuclear reactors.
Cold fission and breakage of nucleon pairs
Most research on nuclear fission is based on the distribution of mass and kinetic energy of fission fragments. However, this distribution is disturbed by the emission of neutrons by the fragments before reaching the detectors.
Although with very low probability, experiments have detected cold fission events, that is, fragments with such a low excitation energy that they do not emit neutrons. However, even in these cases, nucleon pair breakage is observed, manifested as the equal probability of obtaining fragments with an odd or even number of nucleons. The results of these experiments allow a better understanding of the dynamics of nuclear fission up to the point of cleavage, that is, before the nuclear force between the fragments vanishes.
Energy
Contribution

Fission of a heavy nucleus requires a total input energy of about 7 to 8 million electron volts (MeV) to initially overcome the nuclear force that holds the nucleus in a spherical or near-spherical shape, and thereafter to deform it in a two-lobed ('peanut') form in which the lobes are able to keep moving apart from each other, pushed by their mutual positive charge, in the more common process of binary fission (two fission products with positive charge + neutrons). Once the nuclear lobes have been pushed to a critical distance, beyond which the strong short-range force can no longer hold them together, the process of their separation proceeds from the repelling electromagnetic energy between the fragments. The result is two fission fragments hurtling away from each other, at high energy.
About 6 MeV of the input energy from fission is supplied by the simple binding of an extra neutron to the heavy nucleus through the strong force; however, in many fissile isotopes, this amount of energy is not sufficient for fission. Uranium-238, for example, has a near zero fission cross section for neutrons of energy less than one MeV. If no additional energy is provided by any other mechanism, the nucleus will not fission, but will simply absorb the neutron, as occurs when U-238 absorbs slow neutrons and even some fraction of fast neutrons, to become U-239.. The remaining energy to initiate fission can be supplied by two other mechanisms: one is more kinetic energy from the incoming neutron, which is increasingly capable of fissioning a fissile heavy nucleus as it exceeds a kinetic energy of one MeV or more. (the so-called fast neutrons). These high energy neutrons are capable of directly fissioning U-238 (see thermonuclear weapon application, where fast neutrons are supplied by nuclear fusion). However, this process cannot largely occur in a nuclear reactor, since too small a fraction of the fission neutrons produced by any type of fission have enough energy to efficiently fission U-238 (fission neutrons have a modal energy of 2 MeV, but a median of only 0.75 MeV, which means that half of them have less than this insufficient energy).
Among actinide heavy elements, however, those isotopes that have an odd number of neutrons (such as U-235 with 143 neutrons) bind an extra neutron with an extra 1 to 2 MeV of energy on an isotope of the same element with an even number of neutrons (like U-238 with 146 neutrons). This additional binding energy is available as a result of the mechanism of neutron pairing effects. This extra energy is the result of the Pauli exclusion principle that allows an extra neutron to occupy the same nuclear orbital as the last neutron in the nucleus, so that the two form a pair. Therefore, in these isotopes, no kinetic energy of neutrons is needed, since all the necessary energy is supplied by the absorption of any neutron, be it of the slow or fast variety (the former are used in moderated nuclear reactors, and the second in fast neutron reactors and in weapons). As noted above, the subgroup of fissile elements that can be efficiently fissioned with their own fission neutrons (thus potentially causing a nuclear chain reaction in relatively small amounts of the pure material) are termed fissile. Examples of fissile isotopes are uranium-235 and plutonium-239.
Production
Typical fission events release about two hundred million eV (200 MeV) of energy, the equivalent of about >2 trillion Kelvin, in each fission event. The exact isotope that fissions, and whether or not it is fissile or fissile, has only a small impact on the amount of energy released. This can be easily seen by examining the binding energy curve (bottom image), and noting that the average binding energy of actinide nuclides beginning with uranium is about 7.6 MeV per nucleon. Looking further to the left on the binding energy curve, where the fission products cluster, it is easily seen that the binding energy of the fission products tends to center around 8.5 MeV per nucleon. Thus, in any fission event of an isotope in the actinide mass range, approximately 0.9 MeV per nucleon of parent element is released. The fission of U235 by a slow neutron produces almost identical energy to the fission of U238 by a fast neutron. This energy release profile is also valid for thorium and the various minor actinides.
By contrast, most oxidation chemistries, such as burning coal or TNT, release at most a few eVs per event. Therefore, nuclear fuel contains at least ten million times more usable energy per unit mass than chemical fuel. The energy of nuclear fission is released as kinetic energy of the fission products and fragments, and as electromagnetic radiation in the form of gamma rays; In a nuclear reactor, energy is converted to heat when particles and gamma rays collide with the atoms that make up the reactor and its working fluid, usually water or occasionally heavy water or molten salt.
When a uranium nucleus fissions into two daughter nuclei fragments, approximately 0.1 percent of the uranium nucleus's mass appears as the fission energy of ~200 MeV. For uranium-235 (total mean fission energy of 202.79 MeV), typically ~169 MeV appears as the kinetic energy of the daughter nuclei, which separate at 3% of the speed of light, due to the Coulomb repulsion. In addition, an average of 2.5 neutrons are emitted, with an average kinetic energy per neutron of ~2 MeV (total of 4.8 MeV). The fission reaction also releases ~7 MeV in immediate gamma rays, in the form of photons. This last figure means that a nuclear fission explosion or criticality accident emits about 3.5% of its energy in the form of gamma rays, less than 2.5% of its energy in the form of fast neutrons (total of both types of radiation ~ 6%), with the remainder in the form of kinetic energy from the fission fragments (this appears almost immediately when the fragments impact surrounding matter, as simple heat). In an atomic bomb, this heat can serve as to raise the temperature of the bomb's core to 100 million kelvins and cause the secondary emission of soft X-rays, which convert some of this energy into ionizing radiation. However, in nuclear reactors, the kinetic energy of the fission fragments remains in the form of low-temperature heat, which itself causes little or no ionization.
So-called neutron bombs (enhanced radiation weapons) have been built that release a larger fraction of their energy as ionizing radiation (namely, neutrons), but these are all thermonuclear devices that rely on the stage of nuclear fusion to produce extra radiation. The energetic dynamics of pure fission bombs always remains around 6% of the total radiation yield, as an immediate result of fission.
The total energy of the "immediate fission" amounts to about 181 MeV, that is, 89% of the total energy that is finally released by fission over time. The remaining ~11% is released in beta decays that have different half-lives, decays that start immediately after the generation of the fission products; and in delayed gamma emissions associated with these beta decays. For example, in uranium-235 this delayed energy is divided into about 6.5 MeV in beta particles, 8.8 MeV in antineutrinos (released at the same time as the beta particles), and finally an additional 6.3 MeV in emissions. gamma ray emissions of the excited beta decay products (for an average total of 10 gamma ray emissions per fission, in total). Thus, about 6.5% of the total energy of the fission is released some time after the event, as non-immediate or delayed ionizing radiation, with the delayed ionizing energy being split almost equally between gamma and beta ray energy..
In a reactor that has been running for some time, the radioactive fission products will have built up to steady-state concentrations such that their decay rate equals their formation rate, so that their total fractional contribution to the reactor heat (via beta decay) is the same as these fractional radioisotopic contributions to fission energy. Under these conditions, 6.5% of the fission appearing as delayed ionizing radiation (delayed gammas and betas of the radioactive fission products) contributes to the low power steady state reactor heat output. This fraction of production is the one that remains when the reactor is shut down suddenly (suffers a SCRAM). For this reason, the decay heat production of the reactor starts at 6.5% of the steady-state fission power, once the reactor is shut down. However, within a few hours, due to the decay of these isotopes, the decay power is much lower.
The rest of the delayed energy (8.8 MeV/202.5 MeV = 4.3% of the total fission energy) is emitted as antineutrinos, which in practice are not considered "ionizing radiation" 3. 4;. The reason is that the energy released as antineutrinos is not captured by the reactor material as heat, and escapes directly through all materials (including Earth) at nearly the speed of light, and into interplanetary space (the amount absorbed is minuscule). Neutrino radiation is not normally classified as ionizing radiation, because it is almost completely unabsorbed and therefore produces no effect (although the very rare neutrino event is ionizing). Most of the rest of the radiation (6.5% of delayed beta and gamma radiation) ends up being converted to heat in a reactor core or its shielding.
Some processes involving neutrons are characterized by ultimately absorbing or producing energy: for example, the kinetic energy of neutrons does not immediately produce heat if the neutron is captured by a uranium-238 atom to generate plutonium-239, but this energy is emitted if plutonium-239 subsequently fissions. On the other hand, the so-called delayed neutrons emitted as radioactive decay products with half-lives of up to several minutes, coming from the fission daughters, are very important for the control of the reactors, because they give a "reaction time" #3. 4; characteristic for the total nuclear reaction to double, if the reaction is carried out in a zone of delayed criticality that deliberately relies on these neutrons for a supercritical chain reaction (in which each fission cycle produces more neutrons than it absorbs). Without their existence, the nuclear chain reaction would be soon critical and would increase in size faster than could be controlled by human intervention. In this case, the first experimental atomic reactors would have been precipitated into a dangerous and disorderly "immediate critical reaction" before their operators could manually shut them down (for this reason, designer Enrico Fermi included radiation counter control rods, suspended by electromagnets, that could automatically drop into the center of Chicago Pile-1). If these delayed neutrons are captured without fissions, they also produce heat.
Fission induction
The nuclear fission of atoms was discovered in 1938 by the researchers Otto Hahn and Fritz Strassmann from the work carried out by Hahn himself together with Lise Meitner during previous years. For this discovery he received the Nobel Prize in Chemistry in 1944. The study of nuclear fission is considered part of the fields of nuclear chemistry and physics.
- Although fission is practically the disintegration of radioactive matter, often initiated in the easiest way possible (induced), which is the absorption of a free neutron, it can also be induced by throwing other things into a fissionable core. These other things may include protons, other cores, or even high-energy photons in very high amounts (gamma-ray portions).
- Very rarely, a fissionable core will experience spontaneous nuclear fission without an incoming neutron.
- The heavier it is an easier element to induce your fission. Fission in any heavier element than iron produces energy, and fission in any element lighter than iron requires energy. The opposite is also true in nuclear fusion reactions (the fusion of the lightest elements that iron produces energy and the fusion of the heaviest elements that iron requires energy).
- The most frequently used elements to produce nuclear fission are uranium and plutonium. Uranium is the heaviest natural element; plutonium experiences spontaneous disintegrations and has a limited lifetime. Thus, although other elements can be used, these have the best combination of abundance and ease of fission.
Chain Reaction
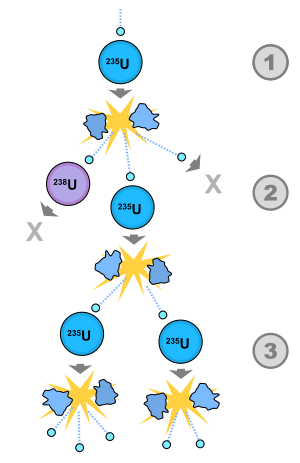
A chain reaction occurs as follows: a fission event begins by releasing 2 or 3 neutrons on average as byproducts. These neutrons fly off in random directions and hit other nuclei, prompting these nuclei to undergo fission. Since each fission event releases 2 or more neutrons, and these neutrons induce further fission, the process speeds up rapidly, causing a chain reaction. The number of neutrons that escape from a quantity of uranium depends on its surface area. Only fissile materials are capable of sustaining a chain reaction without an external neutron source. For the fission chain reaction to take place, it is necessary to adjust the speed of the free neutrons, since if they impact the nucleus of the fissile element with great speed, it may simply pass through or impact it, and it may not. absorb.
Critical mass
The critical mass is the minimum amount of material required for the material to undergo a nuclear chain reaction. The critical mass of a fissile element depends on its density and its physical shape (long rod, cube, sphere, etc.). Since fission neutrons are emitted in random directions, to maximize the chances of a chain reaction, the neutrons should travel as far as possible, thereby maximizing the chances that each neutron will collide with another nucleus. Thus a sphere is the best shape and a flattened sheet is probably the worst, since most of the neutrons would fly off the surface of the sheet and not hit other nuclei.
The density of the material is also important. If the material is gaseous, the neutrons are unlikely to collide with another nucleus because there is too much empty space between the atoms and a neutron would probably fly between them without hitting anything. If the material is put under high pressure, the atoms will be much closer together and the probability of a chain reaction is much higher. High compression can be achieved by putting the material in the center of an implosion, or by throwing a piece of it against another piece of it very strongly (with an explosive charge, for example). A critical mass of material that has started a chain reaction is said to become supercritical.
Moderators
Just getting a lot of uranium in one place isn't enough to start a chain reaction. Neutrons are emitted by a fissioning nucleus at a very high speed. This means that the neutrons will escape from the nucleus before they have a chance to hit any other nucleus (due to a relativistic effect).
A slow moving neutron is called a thermal neutron and only this neutron speed can induce a fission reaction. Thus, we have four neutron speeds:
- A neutron (non-thermal) will quickly escape the material without interaction.
- A medium-speed neutron will be captured by the core and will transform the material into an isotope (but would not induce the fission).
- A slow motion neutron (thermal) will induce a nucleus to experience the fission.
- A really slow mobile neutron will be captured or escaped, but will not cause fission.
Some years before the discovery of fission, the customary way of retarding neutrons was to pass them through a low atomic weight material, such as a hydrogenous material. The slowing down or slowing down process is simply a sequence of elastic collisions between high speed particles and practically resting particles. The more similar the masses of the neutron and the struck particle are, the greater the loss of kinetic energy by the neutron. Therefore the light elements are the most effective as neutron moderators.
A few physicists in the 1930s came up with the possibility of mixing uranium with a moderator: if mixed correctly, high-speed neutrons from fission could be delayed by bouncing off a moderator, with the correct speed, to induce fission in other uranium atoms. The characteristics of a good moderator are: low atomic weight and little or no tendency to absorb neutrons. Possible moderators are then hydrogen, helium, lithium, beryllium, boron and carbon. Lithium and boron absorb neutrons easily, so they are excluded. Helium is difficult to use because it is a gas and does not form any compounds. The choice of moderators would then be between hydrogen, deuterium, beryllium and carbon. It was Enrico Fermi and Leó Szilárd who first proposed the use of graphite (a form of carbon) as a moderator for a chain reaction. Deuterium is the best technologically (introduced into heavy water), however graphite is much cheaper.
Effects of isotopes
Natural uranium is made up of three isotopes: 234U (0.006%), 235U (0.7%), and 238U (99.3%). The rate required for a fission event to occur and not a capture event is different for each isotope.
Uranium-238 tends to capture intermediate speed neutrons, creating 239U, which decays without fission to plutonium-239, which is fissile. Due to their ability to produce fissile material, these types of materials are often called fertile.
High-speed neutrons (52,000 km/s), such as those produced in a tritium-deuterium fusion reaction, can fission uranium-238. However, those produced by the fission of uranium-235, up to 28,000 km/s, tend to rebound inelastically with it, which slows them down. In a nuclear reactor, 238U therefore tends both to slow down the high speed neutrons coming from the fission of uranium-235 and to capture them (with the consequent transmutation to plutonium-239) when their speed moderates.
Uranium-235 fissures with a much wider range of neutron speeds than 238U. Since uranium-238 affects many neutrons without inducing fission, having it in the mix is counterproductive in promoting fission. In fact, the probability of fission of 235U with high speed neutrons may be high enough to make the use of a moderator unnecessary once 238 has been suppressed. U.
However, 235U is present in natural uranium in very small amounts (one part in 140). The relatively small difference in mass between the two isotopes also makes their separation difficult. The possibility of separating the 235U was discovered quite quickly in the Manhattan project, which was of great importance to its success.
Contenido relacionado
Hourglass
Leon Foucault
General relativity


