Non-metal
Non-metals are chemical elements that are not good conductors of electricity and heat. They are very weak, so they cannot be stretched or turned into a sheet.
The chemical properties of non-metals, unlike metals, are very diverse, although they represent a very small number, most of them are essential for biological systems (oxygen, carbon, hydrogen, nitrogen, phosphorus and sulfur). The group of non-metals includes the halogens (fluorine, chlorine, bromine, iodine, astatine and tenesus), which have 7 electrons in their last valence shell, and the noble gases (helium, neon, argon, krypton, xenon, radon), which have 8 electrons in their last shell (except helium, which has 2). Therefore, said layer is complete and they are not very reactive. The rest of the nonmetals belong to various groups and are hydrogen, carbon, sulfur, selenium, nitrogen, oxygen, and phosphorus. Hydrogen's unique properties set it apart from the rest of the elements on the Periodic Table of Elements.
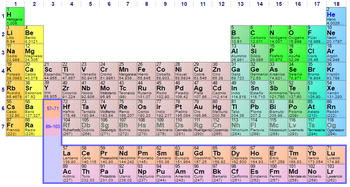
Nonmetals are the elements to the right in the Table, above the broken line for groups 14 to 17 (including hydrogen). Placed in order of increasing atomic number, the elements can be ranked by similarity of properties in 18 families or groups (vertically by columns).
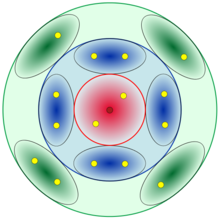
From the point of view of electronics, the elements of a family have the same electronic configuration in the last shell, although they differ in the number of layers (periods). There are 18 groups or families and they correspond to the columns of the Periodic Table of Elements.
Features
Nonmetals vary widely in appearance, are not lustrous, and are generally poor conductors of heat and electricity. Their melting points are lower than those of metals (although diamond, a form of carbon, melts at 3,570 °C). Several nonmetals exist under ordinary conditions as diatomic molecules.
Five gases are included in this list (H2, N2, O2, F2 > and Cl2), a liquid (Br2) and a volatile solid (I2). The rest of the nonmetals are solids that can be hard like diamond or soft like sulfur. Contrary to metals, they are very brittle and cannot be drawn into wires or sheets.
They are found in all three states of matter at room temperature: they are gases (like oxygen), liquids (bromine), and solids (like carbon). They do not have metallic luster and do not reflect light. Many nonmetals are found in all living things: carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur in significant amounts. Others are trace elements: selenium, iodine, chlorine.
They can be solid, liquid or gas, interchangeably. Their melting and boiling points depend on their chemical properties, which are related to their ability to gain electrons (those in the last shell, that is, the valence ones).
They do not conduct electricity well, many of them chemically decompose or recombine before it. With water they generally give acid substances. They are located to the right of the Periodic Table of Elements, and when chemically combined, they gain electrons to acquire the electronic configuration of the noble gas of the same period.
Physical properties:
- Solids (e.g. sulfur and carbon).
- Liquids (only bromine).
- Gaseous (e.g. oxygen and hydrogen).
- They do not possess metallic shine except iodine.
- They're not ductiles or malleable.
- They are not good conductors of heat and electricity (except for some alottropic forms of carbon and phosphorus).
Chemical properties:
- Their atoms have in their last layer 4, 5, 6 and/or 7 electrons.
- By ionizing they acquire negative burden.
- Combined with oxygen form non-metallic or anhydrated oxides.
- They possess molecules formed by two or more atoms.
Reactivity, difference with metals
Nonmetals have a tendency to resemble their closest noble gases in terms of the electronic configuration of their last shell. The less electronegative will have a tendency to lose electrons compared to other more electronegative.
The reactivity of an element measures the tendency to combine with others.
- Variation of reactivity in groups. The groups on the left are more reagent than those on the right because it is easier to lose an electron from the last layer than two, three,... When we get to a certain group the trend is reversed because it will be easier to win the electrons that are missing to look like the nearest noble gas. So, in a period.
- The reactivity of metals increases the more to the left in the period (less electrons to be removed).
- Non-metal reactivity increases by advancing in the period (less electrons to catch).
- Variation of reactivity in groups. As we descend into a group, the electrons of the last layer are further away from the core and, therefore, it will be easier to remove them and, in the case of the Non-Metals, it is more difficult to catch electrons.
- The reactivity of metals increases by advancing in a group (a greater tendency to lose electrons).
- Non-metal reactivity increases the higher in the group (higher tendency to catch electrons)
Lewis Octet Rule
In the formation of compounds there is a tendency to take, lose or share electrons between atoms and thus resemble the electronic configuration of the closest noble gas (eight electrons in the last shell except helium which only has two). This tendency is called the Octet Rule.
The octet rule allows us to explain that metals acquire the noble gas configuration by losing electrons while non-metals acquire it by sharing them.
Contenido relacionado
Azo derivative
Methionine
Balm