Myosin
The myosin is a fibrous protein, whose filaments have a uniform length of 1.6 micrometers and a diameter of 15 nm, which together with actin, mainly allow muscle contraction and intervenes in cell division and vesicle transport.
Myosin is the most abundant protein in skeletal muscle. It represents between 60% and 70% of the total proteins and is the major constituent of the thick filaments.
Structure and composition
Myosin is an ATPase, that is, it hydrolyzes ATP to form ADP and Pi, a reaction that provides muscle contraction.
Myosin is made up of 2 identical heavy chains, each 230 kDa, and 4 light chains, 20 kDa each. The molecule has a double-headed globular region attached to a long double-stranded helical chain. Each head is attached to two different light chains. All myosins have the sequence:
Gly - Glu - Be - Wing - Gly - Lys - Thr[chuckles]required]which is similar to the sequence found in the active site of other ATPases. Lysine binds to the alpha phosphate of ATP.
The uninterrupted α-helical structure of the myosin tail is favored by the absence of proline at intervals of more than 1000 residues and by the abundance of leucine, alanine, and glutamate.
The globular portion of myosin has ATPase activity and combines with actin. Two of the light chains are identical (one on each head) and can be removed without loss of ATPase activity. The other two light chains are not identical and are required for ATPase activity and for the binding of myosin to actin.
Myosin can be cleaved by trypsin into two fragments called meromyosin light and meromyosin heavy.
Light meromyosin forms filaments, lacks ATPase activity, and does not combine with actin; it is an alpha-helical double-stranded chain 850 Å in length. Heavy meromyosin catalyzes ATP hydrolysis, binds to actin, but does not form filaments and generates the force for muscle contraction; It consists of a short rod attached to two globular domains that are the myosin heads. Heavy meromyosin can be cleaved by papain into two rod-shaped subfragments called S2. Each S1 fragment has a site with basic ATP activity and an actin binding site.
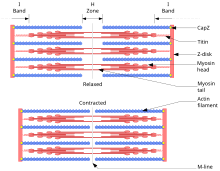
Each myofibril consists of multiple myofilaments, which are thin or thick strands chemically composed of two special proteins, actin and myosin. The myofilaments of a myofibril do not span the entire length of the muscle fiber but are divided into compartments called sarcomeres.
The grouping of thin myofilaments or actin form the light transverse bands of a myofibril and the grouping of the second or myosin, the dark bands. The former are also known as I bands and the latter as A bands. These bands alternate. The I and A bands together are called the sarcomere. In addition to actin, the thin myofilaments contain two other protein molecules, tropomyosin and troponin, which are involved in the regulation of muscle contractions.
One sarcomere is separated from the others by narrow zones of dense material that are the Z lines. The thin filaments are fixed on the Z lines and project in both directions.
In addition to actin, thin myofilaments contain two other types of protein as well as myosin receptor sites. Each myosin filament has small projections called bridges, which when interacting with the actin filaments produce contraction.
The thick myofilaments interdigitate with the free ends of the thin myofilaments and occupy the A band of the sarcomere. In the middle of this band, the H zone can be seen. The myosin molecules that make it up have zones through which they can bind to actin molecules and others, to the AV walking on an Actin F filament).
Types of myosin
At least 18 types of myosin are known:
Myosin type I
Myosin I functions as a monomer and in vesicle transport. It has a step size of 10 nm, and is inferred to be responsible for the adaptive response of the stereocilia in the inner ear.
Myosin type II
Myosin type II drives muscle contraction and cytokinesis. The following properties are observed in this type of myosin:
- The type 2 myosin contains two heavy chains, with an approximate length of 2000 amino acids, and constitute the head and tail of the myosine filament. Each of these heavy chains contains an N-terminal in the head, presenting a thickening in this. While the tail is C-terminal and has a helical structure. These two chains unite forming a spiral, thus obtaining a myosine with two heads.
- The type 2 of myosine also contains four light chains (two per head) that bind both heavy chains by the "neck", that is, the region between the head and the tail. These light chains are often related to essential and regulatory light chains.
Myosin type III
Myosin III is a poorly understood member of the myosin family. It has been studied in vivo in the eyes of Drosophila, where it plays a role in phototransduction. The human homologous gene for myosin III, MYO3A, was discovered thanks to the Human Genome Project and is expressed in the retina and cochlea.
Myosin V type
It is a motor protein of which there are three subclasses (myosin Va, b and c) in mammals. Myosin V is made up of two heavy chains, each with a globular motor domain with ATPase activity linked by the neck region to the domain of the tail. This type of myosin is involved in the intracellular transport of vesicles. The mobility of myosin V is inhibited by calcium. The myosin molecule remains attached to the actin filament, during displacement, by one of its heads.
Contenido relacionado
Noctiluca
Euryarchaeota
Araucariaceae