Molecule
In chemistry, a molecule (from New Latin molecula, which is a diminutive of the word moles, 'mass' 39;) is an electrically neutral and sufficiently stable group of at least two atoms in a defined configuration, held together by strong covalent chemical bonds.
In this strict sense, molecules differ from polyatomic ions. In organic chemistry and biochemistry, the term "molecule" it is used less strictly and is also applied to organic compounds (organic molecules) and biomolecules.
Before, the molecule was defined in a less general and precise way, as the smallest part of a substance that could have an independent and stable existence while still preserving its physicochemical properties. According to this definition, monatomic molecules could exist. In the kinetic theory of gases, the term molecule is applied to any gaseous particle regardless of its composition. According to this definition, the atoms of a noble gas would be considered molecules even though they are composed of unbonded atoms.
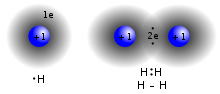
A molecule can consist of several atoms of a single chemical element, as in the case of diatomic oxygen (O2), or of different elements, as in the case of water (H2O). Atoms and complexes held together by non-covalent bonds such as hydrogen bonds or ionic bonds are not usually thought of as individual molecules.
Molecules as components of matter are common in organic substances (and therefore in biochemistry). They also make up most of the oceans and atmosphere. However, a large number of familiar solid substances, including most of the minerals that make up the Earth's crust, mantle, and core, contain many chemical bonds but are not made up of molecules. Furthermore, no typical molecule can be defined in ionic crystals (salts) or covalent crystals, even though they are composed of repeating unit cells, either in one plane (as in graphite) or in three dimensions (as in diamond). or sodium chloride). This system of repeating a unitary structure several times is also valid for most of the condensed phases of matter with metallic bonds, which means that solid metals are not composed of molecules either. In glass (solids that present a disordered glassy state), atoms can also be linked by chemical bonds without any type of molecule being able to be identified, but there is also no regularity of repeating units that characterizes crystals.
Almost all of organic chemistry and much of inorganic chemistry is concerned with the synthesis and reactivity of molecules and molecular compounds. Physical chemistry and, especially, quantum chemistry also study, quantitatively, where appropriate, the properties and reactivity of molecules. Biochemistry is closely related to molecular biology, since both study living beings at the molecular level. The study of specific interactions between molecules, including molecular recognition, is the field of study of supramolecular chemistry. These forces explain physical properties such as solubility or boiling point of a molecular compound.
Molecules are rarely found without interaction with each other, except in rarefied gases and noble gases. Thus, they can be found in crystalline lattices, as in the case of H2O molecules in ice, or with strong interactions, but rapidly changing directionality, as in liquid water. In increasing order of intensity, the most relevant intermolecular forces are: Van der Waals forces and hydrogen bonds.
Molecular dynamics is a method of computer simulation that uses these forces to try to explain the properties of molecules.
A typical molecule cannot be defined for salts or covalent crystals, although these are often composed of repeating unit cells that lie in a plane, for example graphene; or three-dimensionally, for example, diamond, quartz, or sodium chloride. The theme of repeating unit cell structure also applies to most metals that are condensed phases with metallic bonds. Therefore, solid metals are not made of molecules.
In glasses, which are solids that exist in a disordered glassy state, the atoms are held together by chemical bonds without the presence of any definable molecules, nor any of the regular repeating unit cell structure that characterizes salts, covalent crystals and rails.
Molecular Science
The science of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics. Molecular chemistry is concerned with the laws that govern the interaction between molecules that results in the formation and breaking of chemical bonds, while molecular physics is concerned with the laws that govern their structure and properties. In practice, however, this distinction is imprecise. In the molecular sciences, a molecule consists of a stable system (bound state) made up of two or more atoms. Polyatomic ions can sometimes be thought of as electrically charged molecules. The term unstable molecule is used for highly reactive species, that is, short-lived ensembles (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, van complexes. der Waals, or systems of colliding atoms as in the Bose-Einstein condensate.
History and etymology
According to the Royal Spanish Academy, the word «molecule» derives from the Latin moles 'mole' or 'dough' and the diminutive suffix -ula 'small mass'.
- Molécula (1794) - "extremely tiny particle" of French Moléle (1678), New Latin molecule, diminutive Latin moles mass, barrier. A vague meaning at the beginning; the fashion of the word (used until the end of the centuryXVIII only in Latin form) goes back to the philosophy of Descartes.
The definition of a molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules as the smallest particles of pure chemical substance that still retain their composition and chemical properties. This definition is often broken as many substances in ordinary experience, such as rocks, salts, etc., and metals, are made up of large crystalline lattices of chemically bonding atoms or ions, but they are not made of discrete molecules.
Definition and its limits
In a less general and precise way, a molecule has been defined as the smallest part of a chemical substance that retains its chemical properties, and from which the substance can be reconstituted without chemical reactions. According to this definition, which is reasonably useful for those pure substances made up of molecules, there could be "monatomic molecules" of noble gases, while crystal lattices, salts, metals, and most glasses would be left in a confused situation.
Labile molecules can lose their consistency in relatively short times, but if the average lifetime is of the order of a few molecular vibrations, we are in a transition state that cannot be considered a molecule. Currently, it is possible to use pulsed lasers to study the chemistry of these systems.
Entities that share the definition of molecules but have an electrical charge are called polyatomic ions, molecular ions, or ion molecules. Salts composed of polyatomic ions are usually classified as molecular-based materials or molecular materials.
Molecules are made up of particles. A molecule is the smallest piece of matter that still retains the properties of the original matter. Molecules are strongly linked in order to form matter. Molecules are made up of atoms joined by chemical bonds.
A molecule is a unit of substance that can be monatomic or polyatomic. The unit of all gaseous substances is the molecule.
Types of molecules
Molecules can be classified as:
- Discreet molecules: constituted by a well-defined number of atoms, whether of the same element (homonuclear molecules, such as dinitrogen or fullerene) or of different elements (heteronuclear molecules, such as water).
- Macromolecules or polymers: constituted by the repetition of a comparatively simple unit — or a limited set of such units — and which reach relatively high molecular weights.
Links
The atoms that make up molecules are held together by covalent bonds or ionic bonds. Several types of non-metallic elements exist only as molecules in the environment. For example, hydrogen only exists as a hydrogen molecule. A molecule of a compound is made up of two or more elements. A homonuclear molecule is made up of two or more atoms of a single element.
While some people say that a metallic crystal can be considered a single giant molecule held together by metallic bonds, others point out that metals act very differently than molecules.
Covalent
A covalent bond is a chemical bond that involves the exchange of electron pairs between atoms. These pairs of electrons are called shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is called covalent bonding. .
Ionic
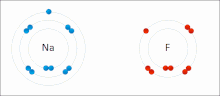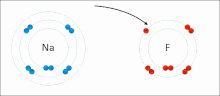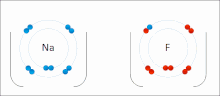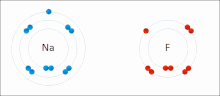
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between ions with opposite electrical charges and is the primary interaction that occurs in ionic compounds. Ions are atoms that have lost one or more electrons (called cations) and atoms that have gained one or more electrons (called anions). This transfer of electrons is called electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a non-metal atom, but these ions can be more complicated in nature, for example molecular ions such as NH4 + or SO4 2−.
At normal temperatures and pressures, ionic bonding creates mostly solids (or occasionally liquids) with no separate identifiable molecules, but vaporization/sublimation of such materials produces small separate molecules where electrons are still transferred enough for bonds to form. are considered ionic rather than covalent.
Description
The molecular structure can be described in different ways. The molecular formula is useful for simple molecules, such as H2O for water or NH3 for ammonia. It contains the symbols of the elements present in the molecule, as well as their proportion indicated by the subscripts.
For more complex molecules, such as those commonly found in organic chemistry, the chemical formula is not enough, and it pays to use a structural formula or skeletal formula, which graphically indicates the spatial arrangement of the various functional groups.
When you want to show various molecular properties, or when dealing with very complex systems such as proteins, DNA or polymers, special representations are used, such as three-dimensional models (physical or represented by computer). In proteins, for example, it is possible to distinguish between primary structure (order of amino acids), secondary (first folding into helices, sheets, turns...), tertiary (folding of the helix/sheet/turn-type structures to give globules) and quaternary (spatial organization between the different globules).
Molecules in quantum theory
Classical mechanics and classical electromagnetism could not explain the existence and stability of molecules, since according to their equations an accelerated electric charge would emit radiation so that electrons would necessarily lose kinetic energy by radiation until they fell on the nucleus atomic. Quantum mechanics provided the first qualitatively correct model that also predicted the existence of stable atoms and provided a very approximate quantitative explanation for empirical phenomena such as the characteristic emission spectra of each chemical element.
In quantum mechanics a molecule or a polyatomic ion is described as a system formed by N{displaystyle N} mass electrons m{displaystyle m} and M{displaystyle M} mass cores mj{displaystyle m_{j}}. In quantum mechanics the physical interactions of these elements are presented by a quantum hamiltonian, whose self-value will be the permitted energies of the system and whose self-functions will describe the molecular orbitals of the molecule, and of these objects the chemical properties of the molecule may be deducted. The following shall be designated through ethe charge of each electron, while that of each core, with Zj{displaystyle Z_{j}} protons, will be Zje{displaystyle Z_{j}e}. To study this system it is necessary to analyze the following quantum hamiltonian:
(1)H^ ^ morl=− − ␡ ␡ j=1N 22m► ► xj2− − ␡ ␡ j=1M 22mj► ► andj2+V(x,and){displaystyle {hat {H}_{mol}=-sum _{j=1}^{N}{frac {hbar ^{2}{2m}{2m}{x}{x}{j}}{2}-sum _{j=1}{j=1}{M}{frac {hbar ^{2m}{2}{2}{2}{x1}}{2}}}}}{2}}}{2}{2}}{x1⁄2}}}}{x1⁄2}}}}}}}}{x1⁄2}}}}}}{x1⁄2}}{x1⁄2}}}{x1⁄2}}}}{x1⁄2}}}}}}}}}}}{x1⁄2}{x1⁄2}}}}}}{x1⁄2}}}}}{x1⁄2}}}{x1⁄2}}}}}}}}}}}}}}{
defined on the space of anti-symtrized functions of integrated square Lsandm2(R3(N+M)){displaystyle L_{sym}^{2}(mathbb {R} ^{3(N+M)}}})}, coordinates associated with electron positions are given by x=(xa! ! 2,...... ,xN)한 한 R3N{displaystyle x=(x_{dot {a}}2,dotsx_{N})in mathbb {R} ^{3N}}} and that of atomic nuclei are given by and=(and1,...... ,andM)한 한 R3M{displaystyle y=(y_{1},dotsy_{M})in mathbb {R} ^{3M}}}. And electrostatic interactions between electrons and nuclei are given by potential V(x,and){displaystyle V(x,y)} you can write as:
(2)V(x,and)=12␡ ␡ iI was. I was. je2 xi− − xj − − ␡ ␡ i,jZje2 xi− − andj +12␡ ␡ iI was. I was. jZiZje2 andi− − andj {displaystyle V(x,y)={fracs {1}{2}}{ineq j}{frac {e^{2}{cHFFFFFF}{i}{i}{i}{i}{i}{i}{i,j}{frac}{i}{i}{i}{i}{i}{i}{i}{i}{i
where the first term represents the interaction of electrons with each other, the second the interaction of electrons with atomic nuclei, and the third the interactions of nuclei with each other. In a neutral molecule it will obviously have that:
␡ ␡ j=1MZj=N{displaystyle sum _{j=1}^{M}Z_{j}=N}
Yeah. M=1{displaystyle M=1} will have a polyelectronic atom if 1}" xmlns="http://www.w3.org/1998/Math/MathML">Z1▪1{displaystyle Z_{1} 20051}1}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/7931a3634a1df66cb4141af88fc9ab5d3804f2eb" style="vertical-align: -0.671ex; width:6.903ex; height:2.509ex;"/>and a hydrogenic atom if Z1=1{displaystyle Z_{1}=1}.
Born-Oppenheimer approximation
Solving the eigenvalues and eigenfunctions problem for the quantum Hamiltonian given by () is a difficult mathematical problem, so it is common to simplify it in some way. So since atomic nuclei are much heavier than electrons (between 103 and 105 times more) it can be assumed that atomic nuclei barely move compared to electrons., so they are considered to be frozen in fixed positions, with which the Hamiltonian () can be approximated by the given Born-Oppenheimer approximation by:
(3)H^ ^ morl,NBO(and)=− − ␡ ␡ j=1N 22m► ► xj2+V(x,and){displaystyle {hat {H}_{mol,N}^{BO}(y)=-sum _{j=}{N}{frac {hbar ^{2}}{2m}}}}{x_{j}{2}{2} +V(x,y)}
defined on the space of functions Lsandm2(R3N){displaystyle L_{sym}^{2}(mathbb {R} ^{3N}}}}} and where and한 한 R3M{displaystyle yin mathbb {R} ^{3M} is the position of the cores that for analysis is considered fixed. The basic result of this analysis is given by the following mathematical result:
|
The property of being self-adjoint will imply that the energies are real quantities, and the fact that they are bounded below will imply that there is a fundamental state of minimum energy below which the electrons cannot decay, and therefore, the molecules will be stable, since electrons cannot lose and lose energy as the equations of classical electromagnetism seemed to predict. Two additional mathematical results tell us what the allowed energies of electrons are within a molecule:
|
In addition, within quantum mechanics it can be shown that positive ions (cations, with a positive charge comparable to the atomic nucleus) can exist, while it is not as easy to have negative ions (anions), the following mathematical result implies has to do with the possibility of cations and anions:
|
Contenido relacionado
Oxidation state
Solubility
Ribonucleic acid