Molecular orbital
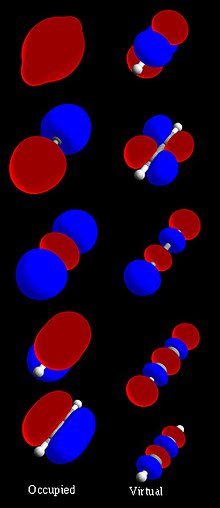
In quantum chemistry, molecular orbitals are regions of space that contain electron density defined by mathematical functions that describe the wavelike behavior of electrons in molecules. These functions can be used to calculate chemical and physical properties such as the probability of finding an electron in a region of space. The term orbital was first introduced in English by Robert S. Mulliken in 1932 as an abbreviation for "one-electron orbital wave function".) from a translation of the German word used in 1925 by Erwin Schrödinger, 'Eigenfunktion'. Since then, the region of space generated with this function has been considered a synonym. Molecular orbitals are usually built by linear combination of atomic orbitals centered on each atom in the molecule. Using electronic structure calculation methods, such as the Hartree-Fock method or the self-consistent field (self-consistent field, SCF), for example, they can be obtained quantitatively.
Electronic configuration
Molecular orbitals are used to specify the electronic configuration of molecules, which allows describing the electronic state of the molecular system as an antisymmetrized product of spin-orbitals. To do this, molecular orbitals are usually represented as a linear combination of atomic orbitals (also called LCAO-MO). One important application is to use approximate molecular orbitals as a simple model to describe bonding in molecules.
Most quantum chemistry methods begin with calculating the molecular orbitals of the system. The molecular orbital describes the behavior of an electron in the electric field generated by the nuclei and an averaged distribution of the rest of the electrons. In the case of two electrons occupying the same orbital, the Pauli exclusion principle requires that they have opposite spins. It should be noted that there are more elaborate methods that do not use the approximation introduced when considering the wavefunction as a product of orbitals, such as the methods based on the use of two-electron (geminal) wavefunctions.
Qualitative obtaining of molecular orbitals
In order to qualitatively describe the molecular structure, molecular orbitals can be obtained by approximating them as a linear combination of atomic orbitals.
Some simple rules that allow us to qualitatively obtain molecular orbitals are:
- The number of molecular orbitals is equal to the number of atomic orbitals included in linear expansion.
- The atomic orbitals mix more (i.e. contribute more to the same molecular orbitals) if they have similar energies. This occurs in the case of homonuclear diatomic molecules such as O2. However, in the event that different nuclei are joined, the unequal load (and therefore the effective load and electronegativity) cause the molecular orbital to deform. In this way the two orbitals 1s of hydrogen overlap 50% contributing equally to the formation of the two molecular orbitals, while in the H-O link the oxygen has a greater coefficient of participation and the molecular orbital will look more like the atomic oxygen orbital (according to the mathematical description of the wave function)
- The atomic orbitals only mix if permitted by the symmetry rules: the orbitals that are transformed according to different irreducible representations of the symmetry group are not mixed. As a result, the most important contributions come from the atomic orbitals that overlap most (link).
The hydrogen molecule
As a simple example, the dihydrogen molecule H2 is illustrative, with two atoms labeled H' and H". The lowest energy atomic orbitals, 1s' and 1s", do not transform according to the symmetry of the molecule. However, the following linear combinations do:
| 1s' - 1s" | Antisimetric combination: denied by reflection, unchanged by other operations |
| 1s' + 1s" | Symmetric combination: unchanged by all operations |
In general, the symmetric combination (called a bonding orbital) is lower in energy than the original orbitals, and the antisymmetric combination (called an antibonding orbital) is higher. Since the dihydrogen molecule H2 has two electrons, both can be described by the bonding orbital, so the system has a lower energy (hence, is more stable) than two hydrogen atoms. free hydrogens. This is known as a covalent bond.
The molecular orbital approximation as linear combination of atomic orbitals (MO-CLOA) was introduced in 1929 by Sir John Lennard-Jones. His publication showed how to derive the electronic structure of difluorine and dioxygen molecules from quantum principles. This quantitative approach to molecular orbital theory represented the birth of modern quantum chemistry.
Types of Molecular Orbitals
When two atoms are bonded, the atomic orbitals merge to give molecular orbitals:
- Linkers: Less energy than any of the atomic orbitals from which it was created. It is in a situation of attraction, that is, in the internuclear region. They contribute to the link in such a way that the positive nuclei overcome the electrostatic forces of repulsion thanks to the attraction of the negative electronic cloud that exists between them up to a given distance that is known as the link length.
- Antilinkers: More energy, and consequently, in a state of repulsion.
The types of molecular orbitals are:
- Orbital σ enlazantes: combination of atomic orbitals s with p (s-s p-p s-p p-s). Links "sencillos" with very small degree of delocalization. Orbitals with cylindrical geometry around the link axis.
- Orbital π enlazantes: combination of perpendicual atomic orbitals to the link axis. Strongly delocalized electrons that interact easily with the environment. They are distributed as electronic clouds above and below the link plane.
- Orbital σ♪ anti-links: excited version (more energy) of the enlazantes.
- Orbitales π♪ anti-links: high-energy orbital π.
- Orbital n: for molecules with heteroatoms (such as N or O, for example). Disappeared electrons do not participate in the link and occupy this orbital.
Molecular orbitals "fill up" of electrons just as atomic orbitals do:
- By increasing order of the energy level: the interlacing orbitals that the anti-linkers are filled before, following among them a growing order of energy. The molecule will tend to fill the orbitals so that the energy situation is favorable.
- Following the Pauli exclusion principle: When molecular orbitals are formed, these can accommodate up to two electrons, having these different spins.
- Applying Hund's maximum multiplicity rule: Degenerated molecular orbitals (with the same level of energy) tend to distribute electrons by separating them to the maximum (parallel spins). This happens to get semi-filled orbitals that are more stable than a full sublayer and another empty due to intense repulsive forces between electrons. This can explain properties of certain molecules such as the paramagnetism of molecular oxygen (the outermost orbital of the molecule has uneven electrons that interact with a magnetic field).
According to these rules, the orbitals are completed. A molecule will be stable if its electrons are mostly in bonding orbitals and unstable if they are in antibonding orbitals:
- By combining two 1s hydrogen orbitals two sigma molecular orbitals are obtained, one enlacing (with less energy) and another
anti-linking (more energy). The two valence electrons are placed with anti-parallel spins in the orbital σ and the orbital σ♪
remains empty: the molecule is stable. - By combining two orbital 1s of helium two sigma molecular orbitals are formed and the four electrons fill all
orbitals. However, the anti-linking orbitals force the molecule to dissociate and become unstable, therefore not
There is He molecule2.
Contenido relacionado
Acrylamide
Euthryptochloa longigulata
Modulated amplitude
