Mole
The mol (symbol: mol) is the unit used to measure the amount of substance, one of the seven fundamental physical quantities of the International System of Units.
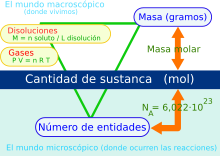
In any substance (element or chemical compound) and considering at the same time a certain type of elemental entities that compose it, the mole, symbol mole, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This figure is the fixed numerical value of Avogadro's constant, when expressed in the unit mol-1, and is called Avogadro's number.
The amount of substance, symbol n, of a system, is a measure of the number of specified elementary entities. An elementary entity can be an atom, a molecule, an ion, an electron, any other particle or a specified group of particles. As a consequence of the definition of the mole, Avogadro's constant and Avogadro's number no longer have an experimental uncertainty in the SI.
Prior to 2019, a mole was defined as the amount of that substance that contains as many elemental entities of the type considered as there are atoms in twelve grams of carbon-12. This definition does not clarify what amount of substance refers to and its interpretation is debated, although it is usually taken for granted that it refers to the number of entities, as seems to be confirmed by the proposal that from As of 2011 the definition is based directly on Avogadro's number (similar to how the meter is defined from the speed of light).
The number of elementary units —atoms, molecules, ions, photons, electrons, radicals or other particles or specific groups of these— existing in a mole of substance is, by definition, a constant that does not depend on the material or the type of particle considered. This quantity is called Avogadro's number (NA) and is equal to:
|
The concept of the mole is of vital importance in chemistry, since, among other things, it allows us to make an infinite number of stoichiometric calculations indicating the existing ratio between reactants and products in chemical reactions. For example: the equation that represents the reaction for the formation of water 2 H2 + O2 → 2 H2O implies that two moles of hydrogen (H2) and one mole of oxygen (O2) react to form two moles of water (H2O).
Another use that should be mentioned is its use to express the concentration in the so-called molarity, which is defined as the moles of the dissolved compound per liter of solution and the molar mass, which is calculated thanks to its equivalence with the atomic mass; vitally important factor to go from moles to grams.
The volume of a gas depends on the pressure, temperature, and number of gas molecules. Different gases under the same conditions have the same kinetic energy. Therefore, two different gases that are at the same temperature and pressure will occupy the same volume. From which it follows that each of them must contain the same number of molecules. And since a mole contains NA molecules, a mole of any gas will have the same volume as a mole of any other gas under the same conditions.
Experimentally it has been determined that the volume occupied by a mole of any gas is 22.4 l under normal conditions. This volume is called the molar volume of the gas. The molar volume is a cube whose edges measure, more or less, 28.2 cm.
History
Given the extremely small size of the fundamental units, and their immensely large number, it is impossible to individually count the particles in a sample. This led to the development of methods to determine these quantities quickly and easily.
The first approach was that of Joseph Loschmidt, trying to quantify the number of molecules in a cubic centimeter of gaseous substances under normal conditions of pressure and temperature.
19th century chemists used a weight-based method as a reference and decided to use mass standards that contained the same number of atoms or molecules. As quantities of the order of the gram are generally used in laboratory experiments, they defined the terms equivalent, gram-atom, gram-molecule, gram-formula, etc., terms that are no longer used, replaced for the mole
Later, the mole is defined in terms of Avogadro's number.
Amadeo Avogadro was born on June 9, 1776, in Turin, Italy. Like his father, he studied law and practiced for three years. However, his true vocation was found in the sciences, for which he dedicated himself to teaching physics at the Vercelli Lyceum and later at the University of Turin.
Inspired by Gay-Lussac's law, which indicates the expansion of gases due to temperature, he thought that if, for example, you have two different volumes of gases and apply the same amount of heat to them, both Volumes will expand to the same degree. And from this reasoning he speculated that this may be because equal volumes of gases contain an equal number of particles.
Seen another way, the hypothesis proposed by Avogadro establishes that all gases with equal volume, pressure and temperature contain the same number of atoms or molecules. This statement was published in the Journal de Physique in 1811. This article pointed out that particles were not necessarily individual atoms, but that these could be combined, forming what he called molecules. breakthrough in understanding the nature of gases:
- It was a rational explanation of Gay-Lussac's law.
- It provided a method for determining the molar masses of gases and thus comparing their densities.
- It provided a solid basis for the development of kinetic-molecular theory.
However, it should be noted that at first his idea was not taken very seriously, since it was opposed to other theories of the time, such as conceiving diatomic gases. Therefore, Avogadro's theory was ignored for almost half a century.
Over time, the evidence leaned in favor of Avogadro's hypothesis. With further research based on X-ray refraction and techniques such as electrolysis, it became even possible to calculate the number of molecules (H2) in two grams of hydrogen, giving the peculiar number of 6,022 141 29 (30) × 1023 which is known as Avogadro's Number.
The term mole was introduced by Wilhelm Ostwald in 1886, who took it from the Latin Mole, which means pile, heap.
Finally, the concept of the Mol was unified in 1971 at the XIV conference on Weights and Measures in Paris, in which the mole was defined as one of the 7 fundamental units of the international system and thus It was adopted by the standards office in the United States and by the IUPAC, being defined as follows:
The mole is the SI unit for measuring amount of substance; which contains as many elementary particles as there are carbon atoms in 0.012 kg of carbon-12. The entity must be specified and can be an atom, a molecule, an ion, an electron, etc.
As of November 16, 2018, the definition of the mole and its pattern were changed, going from being the number of carbon atoms present in 0.012 kg of carbon-12 to the number of atoms present in a perfect sphere of silicon, whose mass is 0.028085 kg.
Clarifications
Since a mol of molecules H2{displaystyle {ce {H2}}} equals 2 grams of hydrogen, a mol of H atoms will then be a gram of this element.
To avoid ambiguities, in the case of elemental macro substances it is therefore appropriate to indicate, where necessary, whether it is atoms or molecules. For example: "a mol of nitrogen molecules" (N2{displaystyle {ce {N2}}}) equals 28 g of nitrogen. Or, in general, specify the type of particles or elementary units referred to.
The mole can be applied to the particles it has in itself, including photons, whose "rest mass" It's void. In this case, comparisons based on mass cannot be made.
Ionic compounds can also use the concept of mol, even if they are not formed by discrete molecules. In that case the mol is equivalent to the term formula-gram. For example: 1 mill NaCl{displaystyle {ce {NaCl}}} (58.5 g) contains NA ions Na+{displaystyle {ce {Na+}}} and NA ions Cl− − {displaystyle {ce {Cl-}}where NA It's Avogadro's number.
- For example, for the case of the water molecule
- It is known that in a molecule H2O{displaystyle {ce {H2O}}} There are two hydrogen atoms and an oxygen atom.
- You can calculate your Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18, i.e. Mr(H)2O) = 18.
- Absolute molecular mass is calculated = 18 × 1.66 × 10−24g = 2.99 × 10−23g.
- Its molar mass is known = M(H2O) = 18 g/mol (1 H mill2Or contains 18 g, consisting of 2 g of H and 16 g of O.
- In a water mill there are 6,02214076 × 1023 H molecules2Or, at the same time:
- In a water mill there are 2 × 6,02214076 × 1023 H atoms (i.e. 2 moles of hydrogen atoms) and 6,02214076 × 1023 O atoms (i.e., 1 mol of oxygen atoms).
As has been said, a certain amount of substance expressed in moles refers to the number of particles (atoms, molecules) that compose it, and not to its magnitude. Just as a dozen grapes contain the same number of fruits as a dozen watermelons, one mole of hydrogen atoms has the same number of atoms as one mole of lead atoms, regardless of the difference in size and weight between them.
Equivalences
- 1 mol of any substance is equivalent to 6,02214076 × 1023 elementary units.
- The mass of a substance mill, called molar mass, is equivalent to the atomic or molecular mass (according to a mol of atoms or molecules) expressed in grams.
- 1 ideal gas mol occupies a volume of 22.4 l to 0 °C of temperature and 1 atm of pressure; and 22.7 l if the pressure is 1 bar (0,9869 atm).
- Number n of moles of atoms (or molecules if it is a compound) present in a quantity of mass substance mIt's:
- n=mMr{displaystyle n={frac {m}{M_{r}}}}}}
where Mr is the relative molar mass, also called the relative molecular mass.
Contenido relacionado
International System Prefixes
Neutrino
Event horizon