Methane
Methane (from the Greek methy vino, and the suffix -ane) is the simplest hydrocarbon alkane, whose chemical formula is CH
4.
Each of the hydrogen atoms is attached to carbon by a covalent bond. It is a nonpolar substance that occurs as a gas at ordinary temperatures and pressures. It is colorless, odorless and insoluble in water.
In nature it is produced as the final product of the anaerobic decomposition of plants. This natural process can be exploited to produce biogas. Many anaerobic microorganisms generate it using the CO
2 as final electron acceptor.
Natural gas contains it in various proportions depending on the deposit from which it is extracted, from 83% to 97%. Commercialized natural gas is mostly methane with some other hydrocarbons added in a small proportion, such as ethane, propane, butane and some nitrogen. In coal mines it is called firedamp and it is very dangerous, since it is easily flammable and explosive. However, in recent decades, the commercial exploitation of coal methane gas as an energy source has gained importance.
Methane is a relatively potent greenhouse gas that contributes to global warming of planet Earth, as it has a global warming potential of 23. This means that in a time measurement of 100 years each kilogram of CH
4 warms the Earth 23 times more than the same mass of CO
2, however, there is approximately 220 times more carbon dioxide in Earth's atmosphere than methane so methane contributes less important to the greenhouse effect.
Potential health (safety) risks
Methane is not toxic. Its main health hazard is the burns it can cause if it ignites. It is highly flammable and can form explosive mixtures with air. Methane reacts violently with oxidizing agents, halogens, and some halogenated compounds. Methane is also an asphyxiant and can displace oxygen in a confined space. Asphyxia can occur if the oxygen concentration is reduced below 19.5% by displacement. The concentrations at which explosive or flammable barriers are formed are much smaller than the concentrations at which the risk of suffocation is significant.[citation needed] If there are structures Built on or near landfills, released methane can seep into buildings and expose occupants to significant levels of methane. Some buildings have systems below their foundations to capture this gas and exhaust it from the building. An example of this type of system is found in the Dakin Building in Brisbane, California.
Reactions
The main reactions of methane are: combustion, reforming with steam (steam reforming) to give synthesis gas (syngas), and halogenation. In general, methane reactions are difficult to control. For example, partial oxidation to methanol is difficult to achieve; the reaction normally proceeds to give carbon dioxide and water.
Combustion
In the combustion of methane a series of steps are involved:
Methane is thought to first react with oxygen to form formaldehyde (HCHO, or H
2CO). The formaldehyde then breaks down into the formyl radical, which then gives carbon dioxide and hydrogen. This process is collectively known as oxidative pyrolysis.
- CH
4 + 2O
2 → CO
2 + 2H
2O
Following oxidative pyrolysis, the H
2 oxidizes to form H
2O, giving off heat. This process is very fast, its usual duration being less than a millisecond.
- 2H
2 + O
2 → 2H
2O
Finally the CO oxidizes, forming CO
2 and releasing more heat. This process is generally slower than the other steps, taking a few milliseconds to complete.
Reformation
The carbon-hydrogen covalent bond is among the strongest of all hydrocarbons, and therefore its use as a raw material is limited. Despite the high activation energy required to break the CH bond, methane is still the main starting material for making hydrogen by steam reforming. The search for catalysts that can facilitate the activation of the CH bond in methane and other light alkanes is a research area of great industrial importance.
Halogenation
Methane reacts with halogens under the right conditions. The reaction takes place as follows.
- CH
4 + X
2 → CH
3X + HX
Where X is a halogen: fluorine (F), chlorine (Cl), bromine (Br) and sometimes iodine (I). The mechanism of this reaction is that of halogenation by free radicals.
Uses
Fuel
Methane is important for power generation as it is used as fuel in gas turbines and steam generators.
Although its heat of combustion, about 802 kJ/mol, is the lowest of all hydrocarbons, if it is divided by its molecular mass (16 g/mol) it is found that methane, the simplest of hydrocarbons, produces more heat per unit mass than other more complex hydrocarbons. In many cities, methane is piped into homes to be used as fuel for heating and cooking. In this context it is called natural gas. In Colombia, as well as in other developing countries, natural gas is used as an alternative fuel for some transportation vehicles.
Industrial uses
Methane is used in industrial chemical processes and can be transported as a refrigerated liquid (liquefied natural gas, or LNG). While a refrigerated container leaks initially heavier than air due to the high density of the cold gas, at room temperature the gas is lighter than air. Gas pipelines transport large quantities of natural gas, of which methane is the main component.
In the chemical industry, methane is the raw material used for the production of hydrogen, methanol, acetic acid, acetic anhydride. When used to make any of these chemicals, methane is first transformed into syngas, a mixture of carbon monoxide and hydrogen, through steam reformation. In this process, methane and water vapor react with the help of a nickel catalyst at high temperatures (700-1100 °C).
- CH
4 + H
2O → CO + 3H
2
The ratio of carbon monoxide to hydrogen can be adjusted by the water gas shift reaction to the desired value.
- CO H
2O → CO
2 + H
2
Other less important chemicals derived from methane are acetylene (made by passing methane through an electric arc) and chloromethanes (chloromethane, dichloromethane, chloroform, and carbon tetrachloride), produced by reacting methane with chlorine gas. However, the use of these products is declining; acetylene is being replaced by cheaper substitutes and chloromethanes due to health and environmental reasons.
Fonts
Natural sources
60% of global emissions are of anthropogenic origin. They come mainly from agricultural activities and other human activities.
The major source of methane is its extraction from geological deposits known as natural gas fields. It is associated with other combustible hydrocarbons and sometimes accompanied by helium and nitrogen. The gas, especially the one located in shallow formations (low pressure), is formed by the anaerobic decomposition of organic matter and the rest is believed to come from the slow degassing of primordial materials located in the deepest parts of the planet, such as This is demonstrated by the presence of up to 7% helium in certain natural gas fields. In general terms, gas deposits are generated in sediments buried deeper and at higher temperatures than those that give rise to oil.
Coal seam methane (CMB) can also be extracted by drilling wells into the coal seams, then pumping the seam water to produce a depressurization that allows desorption of the methane. methane and its rise through the well to the surface. This technique produces 7% of natural gas in the United States, although there may be environmental problems due to declining aquifers and the presence of contaminants in the extracted water.
Methane hydrates or clathrates (combinations of ice and methane on the seafloor) are a potential future source of methane, although no commercial exploitation of them exists so far.
Methanogenesis in a metabolic pathway of anaerobic organisms called methanogenic organisms. The processes in the digestion and defecation of animals (especially cattle): 17%. Bacteria in rice plantations: 12%. Anaerobic digestion of biomass.[citation needed]
Alternative fonts
In addition to natural gas fields, an alternative way to obtain methane is through biogas generated by the fermentation of organic matter found in manure, sewage sludge, domestic garbage, or anywhere other biodegradable raw material, under anaerobic conditions.
Methane can also be obtained industrially using hydrogen (which can be obtained by electrolysis) and carbon dioxide through the Sabatier process as raw materials.
- CO
2 + 4H
2 → CH
4 + 2H
2O.
Methane in the Earth's atmosphere
Methane is a very important greenhouse gas in Earth's atmosphere with a warming potential of 23 over a 100-year period. This implies that the emission of one kilogram of methane will have 23 times the impact of the emission of one kilogram of carbon dioxide over the next hundred years. Methane has a large effect for a short time (about 10 years), while carbon dioxide has a small effect for a long time (about 100 years). Because of this difference in effect and period, the 20-year global warming potential of methane is 63.
The concentration of methane in the atmosphere has increased over the last five thousand years. The most likely explanation for this continued increase lies in the innovations associated with the beginning of agriculture, most likely due to the diversion of rivers for rice irrigation.
Some seven thousand years ago, the technique of irrigation was discovered in the Near East, and later this practice spread to Southeast Asia and South China, thus creating artificial wetlands. In these wetlands, vegetation grew, died, decomposed, and gave off methane.
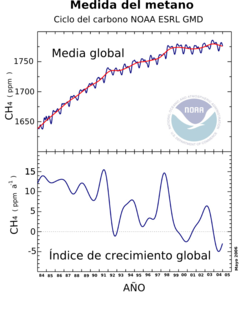
Methane concentration has increased by 150% from 1750 to 1998 and is responsible for 20% of the total radiative forcing of all long-lived and globally distributed greenhouse gases.
The mean concentration of methane at the earth's surface in 1998 was 1,745 ppb and in 2018 it was 1,857.5 ppb. In 2019 Howarth concluded that increasing shale gas production in South America North has contributed significantly to the recent increase in global atmospheric methane.
Its concentration is highest in the northern hemisphere because most sources (natural and anthropogenic) are higher in that hemisphere. Concentrations vary seasonally with a minimum in late summer.
Methane forms near the surface and is carried into the stratosphere by rising air from the tropics. The increase in methane in the Earth's atmosphere is controlled naturally (although human influence can interfere with this regulation) by the reaction of methane with the hydroxyl radical, a molecule formed by the reaction of oxygen with water.
At the beginning of Earth's history, about 3.5 billion years ago, there was a thousand times more methane in the atmosphere than there is today. Primordial methane was released by volcanic activity. It was during this time that life appeared on Earth. Among the first forms of life were methanogenic bacteria that, using hydrogen and carbon dioxide, generated methane and water.
Oxygen was not a major component of the atmosphere until photosynthetic organisms appeared later in Earth's history. Without oxygen, methane could remain in the atmosphere longer, and also in other concentrations, than under current conditions.
Climate change may increase atmospheric methane levels by increasing the release of methane in natural ecosystems.
Methane emissions
Houweling et al. (1999) give the following values for methane emissions (Tg/a: teragrams per year):
| Origin | Emissions of CH4 | ||
|---|---|---|---|
| Mass (Tg/year) | Percentage (%/year) | Total (%/year) | |
| Natural emissions | |||
| Wetlands (including rice pads) | 225 | 83 | 37 |
| Termitas | 20 | 7 | 3 |
| Ocean | 15 | 6 | 3 |
| Hydrates | 10 | 4 | 2 |
| Total Natural | 270 | 100 | 45 |
| anthropogenic emissions | |||
| Energy | 110 | 33 | 18 |
| Basureros | 40 | 12 | 7 |
| Winners (bovines) | 115 | 35 | 19 |
| Waste treatment | 25 | 8 | 4 |
| Biomass combustion | 40 | 12 | 7 |
| Total anthropogenic | 330 | 100 | 55 |
| Submitters | |||
| Sue | 30 | 5 | 5 |
| OH Tropospheric | 510 | 88 | 85 |
| Lost stratospheric | 40 | 7 | 7 |
| Total sinks | 580 | 100 | 97 |
| Emissions - sinks | |||
| Unbalance (trend) | +20 | ~2,78 Tg/ppmm | +7,19 ppmm/a |
Nearly half of the total emission is due to human activity. Plants (eg forests) have recently been identified as a major source of methane. A 2006 paper estimated annual emissions to be 62-236 million tonnes and that this new source could have important implications. However, the authors also noted that their findings were preliminary as to the exact significance of this methane emission. Long-term measurements of methane by NOAA show that the increase in methane in the atmosphere has slowed dramatically after nearly tripling since pre-industrial times. This reduction is believed to be due to declining industrial emissions and the drought in wetland areas.
Sudden release of methane clathrates
At high pressures, such as those found at the bottom of the ocean, methane forms a solid clathrate with water, known as methane hydrate. The amount of methane that is trapped in this way in ocean sediments is unknown, but possibly very large, on the order of a trillion tons.
The "clathrate rifle" is a theory that suggests that if global warming causes these reservoirs to warm enough, all this methane could suddenly be released into the atmosphere. Because methane is twenty-three times more potent (for the same weight, over a 100-year period) than carbon dioxide as a greenhouse gas, it would vastly amplify the greenhouse effect, warming the Earth to unprecedented levels.. This theory would also serve to explain the cause of rapid global warming in the Earth's distant past, such as the Paleocene-Eocene thermal maximum 55 million years ago.
Methane emission from peat bogs
Although less dramatic than clathrates, but already occurring, is an increase in methane emissions from peatlands as permafrost melts. Although permafrost records are limited, in recent years (1999 and 2001) permafrost melting records have been broken in Alaska and Siberia.
Recent measurements in Siberia also show that the methane released is five times higher than previous estimates.
Deletion mechanisms
The main mechanism for removing methane from the atmosphere is through reaction with the hydroxyl radical, which is formed by the bombardment of cosmic rays on water vapor molecules.
- CH
4 + ·OH → ·CH
3 + H
2O
This reaction in the troposphere gives methane a life of 9.6 years. Two more sinks are the soil (160 years of life) and the stratospheric loss due to the reaction with the chemical elements ·OH, Cl and O (2
D) in the stratosphere (120 years life), giving rise to a net life of 8.4 years.
Methane on Mars
The proven presence of methane on Mars remains a mystery and is a possible sign of life on the planet. The seasonal variation of this gas in the Martian atmosphere suggests that there is an active source of geological or biological origin.
Syrtis Major is one of the regions of the red planet where methane originates.
Methane on Mars was discovered in 2003 and appears in the Martian atmosphere at a rate of 10 parts per 1 billion within an atmosphere that is 95% carbon dioxide.
The European probe Mars Express confirmed the permanent presence of methane which, given the photochemical degradation it undergoes, can only be explained if there is a renewable source of this gas.
The origin of the Martian methane can be geological (volcanic, although without evidence of surface volcanoes) or biological. In this second case they should be anaerobic microbes that could perhaps live below the surface in a possible liquid water.
As published in the magazine Science in January 2009, infrared detectors have been used from terrestrial telescopes and it has been possible to observe the evolution of methane over three Martian years (equivalent to 7 terrestrial) and it has been seen that the methane shows variations over time and accumulation in certain regions.
Specifically, it has been seen that the main source contained 19,000 tons with an emission of 600 grams per second.
The half-life of methane on Mars is very short, four Earth years, and may be degraded by oxidants present in airborne dust.
One hypothesis points to the presence of microbes under Martian ice, where radiation could produce hydrogen from liquid water and CO2 provide carbon to ultimately produce methane.
The Martian rover MSL, better known as "Curiosity," is equipped with systems to measure methane and determine what carbon isotope it contains. If it were carbon-12, it would be biological.
Methane sources
The main sources of methane are:
- Decomposition of organic waste by bacteria.
- Natural sources (methanes): 23 %.
- Extraction of fossil fuels: 20% (the methane traditionally burns and emits directly; today it is attempted to store as much as possible to re-use it by producing the so-called natural gas).
- Processes in the digestion and defecation of animals. 17 %(especially cattle).
- Bacteria in rice plantations: 12 %.
- Anaerobic digestion of biomass.
- Plant living matter (we have discovered that plants and trees emit large amounts of methane gas).
60% of global emissions are of anthropogenic origin. They come mainly from agricultural activities and other human activities. The concentration of this gas in the atmosphere has increased from 0.8 to 1.7 ppm, but it is feared that it will do so much more as it is released, as the temperature of the oceans increases, which is stored in the Arctic background.
Properties
- Calories per gram: 12 kcal
- Calories per g CO2: 7.9 kcal
Contenido relacionado
Antoine Lavoisier
Mustard gas
Princess of Asturias Award for Scientific and Technical Research