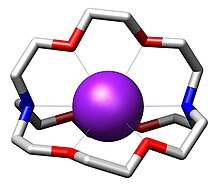
Matter
In classical physics and general chemistry, matter referred to any substance with mass and that occupies space by having volume. This notion of ordinary matter included all everyday objects that touchable are ultimately composed of atoms, made up of interacting subatomic particles. In everyday and scientific usage, the term "matter" usually includes atoms and everything made up of them, as well as any particle (or composite particles). However, this classical notion of matter does not include other forms of matter in the modern physical sense such as massless particles such as photons or other energetic phenomena or waves such as light or heat, which can interact with other forms of matter, and with the detectors and physical instruments used for their measurement.
Introduction
Matter made up of atoms exists in various states (also known as phases). Among them are the classic phases of everyday life, such as solid, liquid, and gas—for example, water exists as ice, liquid water, and gaseous vapor—but other states, such as plasma, are also possible., Bose-Einstein condensates, fermionic condensates, and quark-gluon plasma. Atoms can normally be imagined as a nucleus of protons and neutrons and a surrounding "cloud" of orbiting electrons occupying space. However, this is only somewhat correct because subatomic particles and their properties are governed by their quantum nature, which means that they do not act as everyday objects seem to act, they can act as waves as well as particles. They do not have well-defined sizes or positions. In the Standard Model of particle physics, matter is not a fundamental concept because the elementary constituents of atoms are quantum entities that have no inherent "size" or "volume" in any ordinary sense of the word. Due to the Pauli exclusion principle and other fundamental interactions, some "point particles" known as fermions (quarks, leptons), and many compounds and atoms, are effectively forced to maintain a distance from other particles under everyday conditions; this creates the property of matter that appears to us as matter occupying space. For much of the history of natural science, the exact nature of matter has been contemplated. The idea that matter was made up of discrete building blocks, the so-called "particle theory of matter", appeared independently in ancient Greece and ancient India in the i millennium BCE. C. Among the first philosophers to propose the "particle theory of matter" were find Kanada (ca. VI century BCE or later), Leucippus (~490 BCE), and Democritus (~470‑380 BCE).
Difference Between Matter and Mass
Matter should not be confused with mass, as the two are not the same in modern physics. Matter is a general term that describes any "physical entity" that is distributed throughout space-time and has an associated certain amount of energy. On the contrary, mass is not a substance, but a property of matter and other substances or systems; Several types of mass are defined within physics, including, but not limited to, rest mass, inertial mass, relativistic mass, and mass-energy. Until the 20th century, it was thought that mass was a valid measure for the amount of matter, but quantum field theory, by clarifying the origin of mass, cast doubt on this idea. Thus a proton is 1836 times heavier than an electron, but in reality it is not that a proton has 1836 times more of something than the electron, but rather the intensity of the coupling with the Higgs field of the electron and the quarks that form the protons and neutrons explain a part of the difference in mass, and quantum chromodynamics, by explaining the internal structure of protons and neutrons, clarifies why they are heavier. But in no case is it a greater quantity of something. While there are differing opinions about what should be considered matter, the mass of a substance has exact scientific definitions. Another difference is that matter has an "opposite" called antimatter, but mass has no opposite, that is, there is no "antimass" or negative mass, as far as is known, although scientists dispute the concept. Antimatter has the same mass property (ie positive) as its normal matter counterpart. Different fields of science use the term matter in different and sometimes incompatible ways. Some of these shapes are based on vague historical meanings, from when there was no reason to distinguish mass from mere quantity of matter. There is no single universally agreed scientific meaning of 'matter'. Scientifically, the term "mass" is well defined, but "matter" can be defined in various ways. Sometimes in physics, "matter" is simply equated with particles that have rest mass (that is, they cannot travel at the speed of light), such as quarks and leptons. However, in physics and chemistry, matter exhibits wave-like and particle-like properties, the so-called wave-particle duality (also called "wave-particle duality").
Definition
Based on atoms
A definition of "matter" based on its physical and chemical structure is: matter is made up of atoms. This atomic matter is also sometimes called ordinary matter. For example, deoxyribonucleic acid (DNA) molecules are matter by this definition because they are made up of atoms. This definition can be extended to include charged atoms and molecules, to include plasmas (ion gases) and electrolytes (ionic solutions), which are not included in the definition of atoms. The definition of protons, neutrons and electrons can also be adopted. Until the first third of the XX century it was believed that most of the universe was made up of ordinary matter and photons, however, the discovery of dark matter revealed that most of the matter in galaxies was something that did not appear to be ordinary matter, made up of atoms. The discovery of the accelerating expansion of the universe, around 1998, showed that there actually also existed another exotic form of matter called dark energy (the name is confusing, but it is assumed that it could be a field of matter). As it was, by 2013 detailed measurements from the Planck Surveyor satellite showed that ordinary matter appeared to be only about 5% of all matter in the universe. While the exact composition of dark matter and dark energy is unknown; its effect on the rotation of galaxies and the expansion of the universe allows us to estimate that dark matter accounts for about 27% of the entire universe and dark energy for about 68% of it.
Based on protons, neutrons and electrons
The definition of «matter» is finer than that of atoms and molecules: matter is made up of what atoms and molecules are made of, that is, everything that is formed by positively charged protons, neutral neutrons, and negatively charged electrons. However, this definition goes beyond atoms and molecules, as it includes substances made from these building blocks that not > are simply atoms or molecules, for example, the electron beams of an old cathode ray tube television or the matter of white dwarfs, usually nuclei of carbon and oxygen in a sea of degenerate electrons. At the microscopic level, the constituent "particles" of matter, such as protons, neutrons and electrons, obey the laws of quantum mechanics and present the wave-particle duality. At an even deeper level, protons and neutrons are made up of quarks and the force fields (gluons) that bind them together, leading to the following definition.
Based on quarks and leptons

As seen above, many of the early definitions of what can be called "ordinary matter" were based on its structure or "building blocks". A definition that follows this tradition can be stated at the scale of elementary particles: "ordinary matter is everything that is composed of quarks and leptons," or "ordinary matter is everything that is composed of any elementary fermion except antiquarks and leptons." the antileptons". The connection between these formulations is as follows.
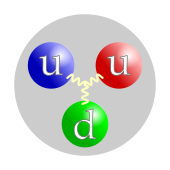
Leptons (most famously the electron) and quarks (which baryons are made of, like protons and neutrons) combine to form atoms, forming molecules. Since atoms and molecules are said to be matter, it is natural to formulate the definition: "ordinary matter is anything made of the same stuff that atoms and molecules are made of." (Note, however, that matter other than atoms and molecules can also be made from these building blocks.) So, since electrons are leptons, and protons and neutrons are made of quarks, this definition, in turn, leads to the definition of matter as "quarks and leptons," which are two of the four types of elementary fermions (the other two are antiquarks and antileptons, which can be considered antimatter as described below). Carithers and Grannis state: "Ordinary matter is composed entirely of first-generation particles, that is, the [up] and [down] quarks, plus the electron and its neutrino." (Higher-generation particles decay rapidly in first-generation particles, so they are not commonly found).
This definition of ordinary matter is more subtle than it appears at first glance. All the particles that make up ordinary matter (leptons and quarks) are elementary fermions, while all the force-carrying particles are elementary bosons. The W and Z bosons that mediate the weak force are not made of quarks or leptons and, therefore, they are not ordinary matter, even though they have mass. In other words, mass is not unique to ordinary matter.
However, the definition of ordinary matter of quarks and leptons identifies the building blocks of matter and includes the compounds made from their components (atoms and molecules, for example). These compounds contain an energy of interaction that holds the constituents together and can make up the majority of the mass of the compound. For example, to a large extent, the mass of an atom is simply the sum of the masses of its component protons, neutrons, and electrons. However, if you dig deeper, protons and neutrons are made up of quarks bound by gluon fields (see quantum chromodynamics dynamics); these gluon fields contribute significantly to the mass of hadrons. In other words, most of what makes up the "mass" of ordinary matter is due to the binding energy of quarks within protons and neutrons. For example, the sum of the masses of the three quarks in a nucleon is about 12.5 MeV/c2, which is small compared to the mass of a nucleon (about 938 MeV/c2). The bottom line is that most of the mass of everyday objects comes from the interaction energy of their elementary components.
The Standard Model groups the particles of matter into three generations, in which each generation consists of two quarks and two leptons. The first generation is formed by the up and down quarks, the electron and the electron neutrino; the second includes the delighted and strange quarks, the muon and the muon neutrino; the third generation is formed by the top and bottom quarks and the tau and the tauon neutrino. The most natural explanation for this would be that the quarks and leptons of higher generations are excited states of the first generations. If this turns out to be so, it would imply that quarks and leptons are composite particles and not elementary particles.
This definition of matter for quarks and leptons also leads to what can be described as "conservation of (net) matter" laws, which will be discussed later. Alternatively, one could return to the mass-volume-space concept of matter, which would lead to the following definition, in which antimatter is included as a subclass Of the mattery.
Based on elementary fermions (mass, volume and space)
A standard or traditional definition of ordinary matter is "anything that has mass and volume (occupies space)". For example, a car would be said to be made of matter, since It has mass and volume (takes up space). The observation that matter occupies space dates back to antiquity. However, the explanation of why matter occupies space is recent. It is argued that it is the result of the phenomenon described in the Pauli exclusion principle, which applies to fermions. Two examples where the exclusion principle relates matter to the occupation of space are white dwarf stars and neutron stars, discussed later.
Thus, mass matter or fermionics can be defined as everything that is composed of elementary fermions. Although we don't encounter them in everyday life, antiquarks (like the antiproton) and antileptons (like the positron) are the antiparticles of the quark and lepton, are also elementary fermions, and have essentially the same properties as quarks and electrons. leptons, including the applicability of the Pauli exclusion principle, which can be said to prevent two particles from being in the same place at the same time (in the same state), that is, it makes each particle "occupy space." This particular definition leads to matter being defined to include anything made of these antimatter particles, as well as the ordinary quark and lepton, and therefore also anything made of mesons, which are unstable particles made up of one quark and one. an antiquark.
On the other hand, bosonic matter, formed fundamentally by photons and other guage bosons, does not satisfy the Pauli exclusion principle and, therefore, does not tend to occupy a volume. Of all this matter, most of it lacks inertial mass, because it is made up of massless bosons such as photons, although weak bosons do have mass, although since they are not fermions they would not occupy volume in the same sense as ordinary matter.
On General Relativity and Cosmology
In the context of relativity, mass is not an additive quantity, in the sense that you cannot add the rest masses of the particles in a system to get the total rest mass of the system. Thus, A more general view of relativity is that it is not the sum of the rest masses, but the energy-momentum tensor that quantifies the amount of matter. This tensor gives the rest mass of the entire system. Therefore, anything that contributes to the energy-momentum of a system is sometimes considered "matter", that is, anything that is not purely gravity. This view is common in fields dealing with general relativity, like cosmology. According to this view, light and other massless particles and fields are part of "matter."
Structure
In particle physics, fermions are particles that obey the Fermi‑Dirac statistics. Fermions can be elemental, like the electron, or compound, like the proton and neutron. The Standard Model has two types of elementary fermions: quarks and leptons, which are discussed below.
Quarks
Quarks are massive particles with spin 1⁄2, which implies that they are fermions. They have an electrical charge of −1⁄3 e (down quarks) or +2⁄3 e (up quarks). For comparison, an electron has a charge of −1 e. They also carry a colored charge, the equivalent of the electrical charge from the strong interaction. Quarks also undergo radioactive decay, which means they are subject to the weak interaction.
| Name | symbol | spine | Electrical charge
(e) | mass
(MeV/c2) | mass comparable to | anti-particle | symbol of the anti-particle |
|---|---|---|---|---|---|---|---|
| quartz of type above (Up) | |||||||
| up.Up) | u | 1♪2 | +2♪3 | 1.5-3.3 | ~ 5 electrons | antiarriba (anti-up) | u |
| enchanted (charm) | c | 1♪2 | +2♪3 | 1160 to 1340 | ~1 proton | anti-enchanted (anticharm) | c |
| summittop) | t | 1♪2 | +2♪3 | 169 100-173 300 | ~180 protons or
~1 volfram atom | anticima (anti-top) | t |
| quartz type down (down) | |||||||
| below (down) | d | 1♪2 | −1♪3 | 3.5 to 6.0 | ~10 electrons | anti-low (anti-down) | d |
| strange (strange) | s | 1♪2 | −1♪3 | 70 to 130 | ~ 200 electrons | anti-foreign (antistrange) | s |
| fund (bottom) | b | 1♪2 | −1♪3 | 4130 to 4370 | ~ 5 protons | anti-phony (antibottom) | b |
Baryonics
Baryons are strongly interacting fermions and are subject to Fermi‑Dirac statistics. Among the baryons are protons and neutrons, which are found in atomic nuclei. However, many other unstable baryons also exist. The term baryon usually refers to triquarks, particles made up of three quarks. Also, "exotic" baryons made up of four quarks and one antiquark are known as pentaquarks, but their existence is not generally accepted.
Baryonic matter is the part of the universe made up of baryons (including all atoms). This part of the universe does not include dark energy, dark matter, black holes, or various forms of degenerate matter, such as those that make up white dwarf stars and neutron stars. The microwave light observed by the Wilkinson Microwave Anisotropy Probe suggests that only about 4.6% of the part of the universe that is within reach of the best telescopes (that is, the matter that can be visible because light could reach us from ella) is made up of baryonic matter. About 26.8% is dark matter, and about 68.3% is dark energy.
The vast majority of the ordinary matter in the universe is unseen, as visible stars and gas within galaxies and clusters account for less than 10% of the ordinary matter contribution to mass-energy density of the universe.
Hadronics
Hadronic matter can refer to "ordinary" baryonic matter made up of hadrons (baryons and mesons) or QCD matter (a generalization of atomic nuclei), i.e. "low" temperature CDC matter. Includes degenerate matter and the result of collisions of high-energy heavy nuclei.
Degenerate
In physics, degenerate matter refers to the ground state of a fermion gas at a temperature close to absolute zero. The Pauli exclusion principle requires that only two fermions can occupy a state quantum, one with spin up and one with spin down. Therefore, at zero temperature, the fermions fill enough levels to accommodate all available fermions, and in the case of many fermions, the maximum kinetic energy (called the Fermi energy) and the gas pressure become very large and depend of the number of fermions rather than the temperature, unlike the normal states of matter.
Degenerate matter is believed to be produced during the evolution of heavy stars. Subrahmanyan Chandrasekhar's demonstration that white dwarf stars have a maximum allowable mass due to the exclusion principle sparked a revolution in evolutionary theory stellar.
Degenerate matter includes the part of the universe made up of neutron stars and white dwarf stars.
Strange
Strange matter is a particular form of quark matter, often thought of as a liquid of up, down, and strange quarks. It is contrasted with nuclear matter, a liquid of neutrons and protons (made up of up and down quarks), and non-strange quark matter, which is a quark liquid containing only up and down quarks. At a high enough density, the foreign matter is expected to be a superconductor of color. The hypothesis is that the strange matter is found in the core of neutron stars or, more speculatively, in the form of isolated droplets that can vary in size from femtometers (strangelets) to kilometers (quark star).
Two meanings
In particle physics and astrophysics, the term is used in two ways, one more broad and one more specific.
- The wider meaning is simply quartz matter which contains three quartz flavors: above, below and strange. In this definition there is critical pressure and associated critical density. When nuclear matter (formed by protons and neutrons) is compressed beyond this density, protons and neutrons are dissociated in quaarks, giving rise to quartz matter (probably strange matter).
- The most restricted meaning is that of quartz matter, which is more stable than nuclear. The idea that this could happen is the "hypothesis of the strange matter" of Bodmer and Witten. In this definition, critical pressure is zero: the real basic state of matter is always. quartz matter. The nuclei we see in the matter that surrounds us, which are droplets of nuclear matter, are metastatic and, given sufficient time (or adequate external stimulus), would decay in droplets of foreign matter, that is, strange.
Leptons
Leptons are particles with spin 1⁄2, which means they are fermions. They have an electrical charge of −1 e (charged leptons) or 0 e (neutrinos). Unlike quarks, leptons do not have a color charge, which means they do not undergo the strong interaction. Leptons also undergo radioactive decay, which means they are subject to the weak interaction. Leptons are massive particles; so they are subject to gravity.
| Name | symbol | spine | electric charge
(e) | mass
(MeV/c2) | mass comparable to that of | anti-particle | symbol of the anti-particle |
|---|---|---|---|---|---|---|---|
| leptons loaded | |||||||
| electron | e− | 1♪2 | −1 | 0,5110 | 1 electron | anti-electronic | e+ |
| muon | μ− | 1♪2 | −1 | 105.7 | ~ 200 electrons | antimuon | μ+ |
| tau | Δ− | 1♪2 | −1 | 1777 | ~ 2 protons | antibola | Δ+ |
| neutrinos | |||||||
| electronic neutrino | . e | 1♪2 | 0 | 40.000 460 | . 1♪1000 electron | antineutrino | . e |
| muonic neutrino | . μ | 1♪2 | 0 | 0.19 | . 1♪2 electron | atineutrino muonic | . μ |
| tauonic neutrino | . Δ | 1♪2 | 0 | 18.2 | 40 electrons | tauonic antineutrino | . Δ |
Bosons
In addition to leptons and quartz, the most abundant particle type in the universe would be photons, which according to recent estimates would be in total some 4⋅ ⋅ 1084{displaystyle 4cdot 10^{84}. In fact, it is estimated that there are about a billion (r=109{displaystyle r=10^{9}}) photons for every barion in the universe. More exactly the number of photons in relation to the number of varions is given by:
n=1r≡ ≡ NbNγ γ 2.75× × 10− − 8Ω Ω bh2{displaystyle n={frac {1}{r}}{equiv {frac {N_{b}}}{N_{gamma }}}simeq 2.75times 10^{-8}Omega {_{b}}}}}{{2}}}{
where the last term was estimated from the last measurements of the scientific satellite Planck Surveyor (2018) as Ω Ω bh2=0.0224± ± 0.0001{displaystyle Omega {_{b}}h^{2}=0.0224pm 0.0001}.
They are pure substances that are made up of 2 or more elements combined in fixed proportions. Compounds can be broken down by chemical processes into their constituent elements. Example: Water, with the formula H2O, is made up of the elements hydrogen (H) and oxygen (O) and can be broken down into these by the action of an electric current (electrolysis).. Compounds are represented by chemical formulas that specify the elements that make up the compound and the number of atoms of each that make up the molecule. Example: In water there are 2 atoms of the element hydrogen and 1 atom of the element oxygen forming the molecule
TYPES:
Heterogeneous mixtures: These are mixtures in which the components can be distinguished with the naked eye. Example: Water with oil, granite, sand in water, etc.Heterogeneous mixtures: These are mixtures in which the components can be distinguished with the naked eye. Example: Water with oil, granite, sand in water, etc.
- Homogeneous mixtures: Also called Dissolutions. They are mixtures in which their components cannot be distinguished with the naked eye. Example: Dissolution of salt in water, air, an alloy of gold and copper, etc.

In bulk, matter can exist in various forms or states of aggregation, known as phases, depending on ambient pressure, temperature, and volume. A phase is a form of matter with uniform chemical composition and physical properties (such as density, specific heat, refractive index, etc.). These phases include the three known ones (solids, liquids and gases) and more exotic states of matter (such as plasmas, superfluids, supersolids, Bose‑Einstein condensates,…). A fluid can be a liquid, a gas or a plasma. There are also paramagnetic and ferromagnetic phases of magnetic materials. By changing conditions, matter can pass from one phase to another. These phenomena are called phase transitions and are studied in thermodynamics. In nanomaterials, the enormous increase in surface area to volume ratio results in matter that can have totally different properties than the bulk material and are not well described by any bulk phase.
Phases are sometimes called states of matter, but this term can be confused with thermodynamic states. For example, two gases maintained at different pressures are in different thermodynamic states (different pressures), but in the same phase (both are gases).
Antimatter
Antimatter is a matter made up of the antiparticles of those that make up ordinary matter. If a particle and its antiparticle come into contact, they both annihilate; that is, they can become other particles with the same energy according to Albert Einstein's E = mc2 equation. These new particles can be high-energy photons (gamma rays) or other particle-antiparticle pairs. The resulting particles are endowed with an amount of kinetic energy equal to the difference between the rest mass of the annihilation products and the rest mass of the original particle-antiparticle pair, which is usually quite large. Depending on the definition of "matter" one adopts, antimatter can be said to be a particular subclass of matter or the opposite of matter.
Antimatter does not occur naturally on Earth, except very briefly and in small amounts (as a result of radioactive decay, lightning, or cosmic rays). This is because any antimatter that came to exist on Earth outside the confines of a proper physics laboratory would almost instantly encounter the ordinary matter that Earth is made of and annihilate itself. Antiparticles and some stable antimatter (such as antihydrogen) can be made in trace amounts, but not enough to do more than prove some of their theoretical properties.
There is much speculation in science and science fiction about why the observable universe is almost entirely matter (in the sense of quarks and leptons, but not antiquarks or antileptons), and whether other places are instead almost entirely matter. antimatter (antiquarks and antileptons). It is believed that in the early universe matter and antimatter were equally represented. The disappearance of antimatter requires an asymmetry in physical laws called CP (charge-parity) symmetry violation, which can be obtained from the Standard Model. However, at this time, the apparent asymmetry of matter and antimatter in the visible universe is one of the important unsolved problems in physics. The possible processes by which it was produced are studied in more detail in the baryogenesis section.
Antimatter particles can be defined by their negative baryon or lepton number. In contrast, particles of "normal" matter (not antimatter) have a positive baryon or lepton number. These two classes of particles are the antiparticle pair of each other.
In October 2017 scientists reported new evidence that matter and antimatter, likewise produced in the Big Bang, are identical, should annihilate each other, and as a result, the universe should not exist. This implies that something unknown to scientists must have stopped the complete mutual destruction of matter and antimatter in the early-forming universe or that it led to an imbalance between the two forms.
Conservation
NO conservation of mass
In chemistry and the phenomena of classical physics, mass is a conserved quantity. However, when considering nuclear physics phenomena such as fusion, fission, or particle disintegration, mass is not strictly conserved. Therefore, neither in relativistic physics nor in quantum physics there is a law of conservation of mass.
Baryon and lepton conservation
Even so, two magnitudes associated with the "amount of matter" can be defined, in the quark-lepton sense (and antimatter in the antiquark-antilepton sense), which do seem to be conserved experimentally are: baryon number and lepton number, are conserved in the standard model of particle physics. A baryon, like a proton or a neutron, has a baryon number of one, and a quark, since there are three in a baryon, has a baryon number of 1/3. Thus the net amount of matter, measured by the number of quarks (minus the number of antiquarks, each with a baryon number of −1/3), is proportional to the baryon number. The number of leptons (minus the antileptons), called the lepton number, is practically impossible to change in any process. Even in a nuclear bomb, none of the baryons (protons and neutrons that make up atomic nuclei) are destroyed. There are as many baryons after the reaction as before, so none of these particles of matter are destroyed. None are converted to non-material particles (such as photons of light or radiation). Instead, nuclear (and perhaps chromodynamic) binding energy is released, as these baryons coalesce into medium-sized nuclei that have less energy (and, equivalently, less mass) per nucleon compared to the original small nuclei. (hydrogen) and large (plutonium, etc.). Even in electron-positron annihilation, there is no net matter to be destroyed because there was initially zero net matter (zero total lepton numbers and baryon numbers) before the annihilation—one lepton minus one antilepton equals zero net lepton numbers— and this net amount of matter does not change, as it simply remains zero after annihilation.
In contrast, the number of bosons is not conserved, these being their own antiparticles can be generated or annihilated by interaction between them, without there being a constant bosonic number. Therefore, the only consistent way to define a fixed amount of matter would be in terms of baryon and lepton numbers. Baryons and leptons can be created, but antibaryons or antileptons accompany their creation; they can be destroyed by killing them with antibaryons or antileptons. Since antibaryon/antilepton numbers have negative baryon/leptonic numbers, the total baryon/leptonic numbers are unchanged, so matter is conserved. However, both baryon/leptons and antibaryon/antileptons have positive mass, so the total amount of mass is not conserved. Also, outside of natural or man-made nuclear reactions, there is almost no generally available antimatter in the universe (see baryon asymmetry and leptogenesis), so particle annihilation is rare under normal circumstances.
Dark matria and energy
Ordinary matter, in the definition of quarks and leptons, makes up about 4% of the energy in the observable universe. The rest of the energy is theorized to be due to exotic forms, of which 23% is dark matter, and 73% is dark energy.
Dark Matter
In astrophysics and cosmology, dark matter is matter of unknown composition that neither emits nor reflects enough electromagnetic radiation to be directly observed, but whose presence can be inferred from gravitational effects on Earth. visible matter. Observational evidence for the early universe and the Big Bang theory require that this matter have energy and mass, but not that it be composed of ordinary baryons (protons and neutrons). The commonly accepted view is that most dark matter is non-baryonic. As such, it is composed of particles not yet observed in the laboratory. Perhaps they are supersymmetric particles, not Standard Model particles, but relics formed at very high energies in the early phase of the universe and still around.
Dark Energy
In cosmology, dark energy refers to the source of the repelling influence that accelerates the rate of expansion of the universe. Its exact nature is a mystery, although its effects can be reasonably modeled by assigning matter-like properties to a vacuum, such as energy density and pressure.
«(trad.) 70% of the material density of the universe seems to be in the form of dark energy. Twenty-six percent is dark matter. Only 4 % is ordinary matter. Thus, less than one part of every 20 is made up of the matter that we have experimentally observed or described in the standard model of particle physics. Of the other 96 %, apart from the above-mentioned properties, we know absolutely nothing."Lee Smolin (2007), The Trouble with Physicsp. 16
Philosophical concept of matter
Since the beginning of philosophy, and in almost all cultures, this concept is found loosely formulated as what remains beneath the changing appearances of things in nature. According to this idea, everything observable is given in its diverse and changing appearances in a support or entity in which the movement and change of things resides: matter.
Unique or diverse principle of matter
An important philosophical question was whether all matter or material substratum had a single beginning or had multiple sources. That said substratum is a single or several material principles (air, fire, earth and water), was a question raised by the Milesian philosophers; the Eleatians, on the other hand, questioned the reality of movement and, together with the Pythagoreans, based being on a formal principle of thought, leaving matter merely as something indeterminate and inconsistent, a non-being.
The atomic theory of matter
The atomistic theory of antiquity had greater historical significance, reinstated by rationalist mechanism in the 17th century and XVIII, which provided the basic theoretical support for the birth of modern physical science.
Hilemorphism
Plato and above all Aristotle elaborated the concept of form, correlative and in opposition to matter, giving it the metaphysical and problematic character that it has had throughout the history of thought, at the same time that it has served as a concept that applies in other contexts.
It was Aristotle who elaborated the concept of matter in a more complete way, although the metaphysical aspect was relegated to scholasticism.
For Aristotle, following the tradition of the Milesians and Plato, the fundamental characteristic of matter is the receptivity of form. Matter can be anything capable of receiving a form. For this reason, first of all, matter is the potential to be something, the something being determined by the form.
Based on this concept, there are as many classes of matter as there are classes of forms capable of determining a being. Since the movement consists of a change in the form of the substance, the movement is explained in terms of matter as power and the act as a way of determining the substance.
Matter, as substance and subject, is the very possibility of movement. There are as many classes of matter as there are possible determinations of the substance in its predicates.
When the determinations are accidental, the matter is given by the situation of the potential substance with respect to receiving a new form. Thus, sitting in act is potential matter for standing; the movement consists of going from being standing in power, to being standing in act.
The problem is the explanation of the substantial change that occurs in the generation and corruption of the substance. Here appears the metaphysical concept of raw material, pure power of being that is nothing, since it has no form of determination.
The traditional scholastic formula by which raw material is usually defined gives the idea that it is really difficult to conceive a reality that corresponds to said concept: It is not a what (substance), nor a quality, nor a quantity or anything else by which being is determined. A merely negative definition that violates the very laws of definition.
Materialism
Materialism is the idea that matter is primary and that consciousness exists as a consequence of a highly organized state of it, which produces a qualitative change.
Regarding the relationship of human thought and the world around us and the knowability of that world, materialism asserts that the world is material and that it exists objectively, independently of consciousness. According to this conception, consciousness and thought develop from a higher level of organization of matter, in a process of reflection of objective reality.
Materialism also maintains that matter has not been created out of nothing, but exists in eternity and that the world and its regularities are knowable by humans, since it is possible to demonstrate the accuracy of this way of conceiving a natural process, reproducing it ourselves, creating it as a result of its own conditions and also putting it at the service of our own ends, destroying the "thing in itself, unattainable".
Aggregation States
Antoine-Laurent de Lavoisier's Elementary Treatise on Chemistry (1789) mentions the three states of aggregation of matter known until before the discovery of plasma in the XIX: the solid, the liquid and the "elastic and aeriform" state The word "gas" was invented by Jan Baptista van Helmont in 1648 to name what was previously known as "air". This use can still be seen in 1774, for example, in Joseph Priestley's Experiments and Observations on Different Kinds of Air, but in Lavoisier's treatise the term "gas" it is used extensively. Plasma was discovered in 1879 by William Crookes, who called it "radiant matter". The term "plasma" it would be used for the first time in 1929 by Irving Langmuir. Between 1924 and 1925 the fifth state of matter was predicted by Satyendra Nath Bose and Albert Einstein, for which it would be called the Bose‑Einstein condensate. The Fermi quantum gas was first created in 1999 at JILA at the University of Colorado, as was the fermionic molecular condensate in 2003.
Law of Conservation of Matter
As a scientific fact, the idea that mass is conserved goes back to the chemist Lavoisier, the French scientist considered the father of modern chemistry who carefully measured the mass of substances before and after taking part in a chemical reaction, and arrived at the conclusion that matter, measured by mass, is neither created nor destroyed, but only transformed in the course of reactions. His conclusions are summarized in the following statement: In a chemical reaction, matter is neither created nor destroyed, it is only transformed. The same principle was discovered earlier by Mikhail Lomonosov, so it is sometimes quoted as Lomonosov-Lavoisier's law, more or less in the following terms: The mass of a system of substances is constant, with independence of the internal processes that may affect it, that is, "The sum of the products is equal to the sum of the reactants, keeping the mass constant". However, both modern techniques and the improvement of the precision of the measurements have made it possible to establish that the Lomonosov-Lavoisier law is fulfilled only approximately.
The equivalence between mass and energy, discovered by Einstein, forces us to reject the statement that conventional mass is conserved, because mass and energy are mutually convertible. In this way it can be affirmed that the equivalent relativistic mass (the total of material mass and energy) is conserved, but the rest mass can change, as it happens in those relativistic processes in which a part of the matter is converted into photons. The conversion in nuclear reactions of a part of the matter into radiant energy, with a decrease in the rest mass, is observed, for example, in fission processes such as the explosion of an atomic bomb, or in fusion processes such as the constant emission of energy what the stars do.
Distribution of matter in the universe
According to current physical models, only about 5% of our universe is made up of ordinary mass matter. It is assumed that a significant part of this mass would be baryonic matter made up of baryons and electrons, which would only be about 1/1850 of the mass of baryonic matter. The rest of our universe would be made up of dark matter (23%) and dark energy (72%).
Even though baryonic matter makes up such a small percentage, half of it has yet to be found. All observable stars, galaxies, and gas form less than half the baryons that there should be. The main hypothesis about the rest of baryonic matter not found is that, as a consequence of the process of formation of structures after the big bang, it is distributed in low-density gaseous filaments that form a network throughout the universe and in whose nodes are the various clusters of galaxies. Recently (May 2008) the XMM-Newton telescope of the European space agency has found evidence of the existence of such a network of filaments.
Intrinsic properties of matter
Mass
In physics, mass (from the Latin massa) is a magnitude that expresses the amount of matter in a body, measured by its inertia, which determines the acceleration produced by a force acting on it. It is an intrinsic property of bodies that determines the measure of inertial mass and gravitational mass. The unit used to measure mass in the International System of Units is the kilogram (kg).
Non-mass matter
A large part of the energy in the universe corresponds to forms of matter made up of particles or fields that do not have mass, such as light and electromagnetic radiation, both made up of photons. Along with these non-mass particles, the existence of other particles such as the graviton, photino and gravitino is postulated, which would all be massless particles although they contribute to the total energy of the universe.
Mass matter
The mass matter is organized into several levels and sublevels. The mass matter can be studied from the macroscopic and microscopic points of view. Depending on the level of description adopted, we must adopt classical descriptions or quantum descriptions. A part of the mass matter, specifically that which makes up subcooled bodies and stars, is made up of molecules, atoms, and ions. When the temperature conditions allow it, the matter is condensed.
Electric charge
The electrical charge is an intrinsic physical property of some subatomic particles that is manifested by forces of attraction and repulsion between them through electromagnetic fields. Electrically charged matter is influenced by electromagnetic fields, being, in turn, a generator of them. The so-called electromagnetic interaction between charge and electric field is one of the four fundamental interactions of physics. From the point of view of the standard model, electric charge is a measure of the ability of a particle to exchange photons.
One of the main characteristics of electric charge is that, in any physical process, the total charge of an isolated system is always conserved. That is, the algebraic sum of the positive and negative charges does not vary in time.
Electric charge is discrete in nature, a phenomenon experimentally demonstrated by Robert Millikan. For historical reasons, electrons were assigned a negative charge: −1, also expressed as −e. Protons have a positive charge: +1 or +e. Quarks are assigned fractional charge: ±1/3 or ±2/3, although they have not been observed free in nature.
Current research in physics suggests that electric charge is a quantized property. The most elementary unit of charge was found to be the charge that the electron has, that is, around 1.602 176 487(40) × 10−19 coulombs (C) and is known as elementary charge. The value of the electric charge of a body, represented as q or Q, is measured according to the number of electrons it possesses in excess or deficiency.
Impenetrability
In physics, impenetrability (from impenetrable) is the resistance that a portion of matter opposes to another occupying its same place in space. No body can simultaneously occupy the place of another. Likewise, impenetrability is the resistance that a body opposes to being pierced. It is in the general property category. It should be noted that impenetrability refers to the ability of ordinary matter not to be penetrated by fragments of ordinary matter. This is important since, for example, ordinary matter can be easily penetrated by non-ordinary matter particles such as neutrinos, which can pass through large layers of matter without interacting with it.
Going back to the case of ordinary matter, impenetrability depends on the Pauli exclusion principle by which electrons, as fermionic particles that they are, are forced to occupy different layers, thus making a stable atom a structure with wide extension in space. When two fragments of ordinary matter approach each other, the respective atoms come closer, but due to the restriction imposed by the Pauli principle, their electron clouds cannot interpenetrate, resulting in an effective repulsion. This is, ultimately, the cause of the impenetrability of ordinary matter.
Wave-particle duality
According to classical physics there are clear differences between wave and particle. A particle has a defined position in space and has mass while a wave extends in space, characterized by having a defined speed and zero mass. The wave-particle duality, also called wave-particle duality is a quantum phenomenon, well verified empirically, by which many particles can exhibit typical wave behavior in experiments while they appear as compact and localized particles in other experiments. Given this dual behavior, it is typical of quantum mechanical objects, where some particles can present very localized interactions and, like waves, they exhibit the phenomenon of interference.
Wave-particle duality is currently considered to be a “quantum mechanical concept that there are no fundamental differences between particles and waves: particles can behave like waves and vice versa.” (Stephen Hawking, 2001)
This is a fact proven experimentally on multiple occasions. It was introduced by Louis‑Victor de Broglie, a French physicist of the early XX century. In 1924 in his doctoral thesis, inspired by experiments on the diffraction of electrons, he proposed the existence of matter waves, that is to say that all matter had a wave associated with it. This revolutionary idea, based on the analogy with which radiation had an associated particle, a property already demonstrated at the time, did not arouse great interest, despite the correctness of his statements, since there was no evidence of its occurrence. However Einstein recognized the importance of it as a result of his results of the experiments of the photoelectric effect. In 1905, the same year that he formulated his theory of special relativity, Albert Einstein proposed a mathematical description of this phenomenon that seemed to work correctly and in which the emission of electrons was produced by the absorption of quanta of light that would later be called photons. In an article titled "A heuristic point of view on the production and transformation of light" he showed how the idea of discrete particles of light could explain the photoelectric effect and the presence of a characteristic frequency for each material below which no effect occurred. For this explanation of the photoelectric effect, Einstein would receive the Nobel Prize in Physics in 1921. In 1929 De Broglie received the Nobel Prize in Physics for his work.
Antimatter
Most of the particles in nature correspond to an antiparticle that has the same mass, the same spin, but the opposite electric charge. Some particles are identical to their antiparticle, such as the photon, which has no charge. But not all charged neutral particles are identical to their antiparticle. We have always had the impression that the laws of nature seemed to have been designed so that everything was symmetric between particles and antiparticles until experiments called CP violation (charge-parity violation) found that time symmetry was violated in certain events. of the nature. The observed excess of baryons relative to anti-baryons in the universe is one of the major unanswered problems in cosmology.
Particle-antiparticle pairs can annihilate each other if they are in the appropriate quantum state. These states can occur in various processes. These processes are used in particle accelerators to create new particles and test the theories of particle physics. High energy processes in nature can create antiparticles, and these are visible due to cosmic rays and in certain nuclear reactions. The word antimatter refers to elementary antiparticles, the antiparticle compounds made from them (such as antihydrogen), and larger formations that can be made from them.
antimatter is the extension of the concept of antiparticle to matter. Thus, antimatter is a less frequent form of matter that is made up of antiparticles, as opposed to ordinary matter, which is made up of particles. For example, an antielectron (an electron with a charge also called a positron) and an antiproton (a negatively charged proton) could form an antimatter atom, in the same way that an electron and a proton form a hydrogen atom. The contact between matter and antimatter causes their mutual annihilation; this does not mean its destruction, but a transformation that gives rise to high-energy photons, which produce gamma rays and other particle-antiparticle pairs.
Spin
The spin (from English spin 'spin, turn') is a physical property of elementary particles by which they have an intrinsic angular momentum of fixed value. Spin was introduced in 1925 by Ralph Kronig and independently by George Uhlenbeck and Samuel Goudsmit. The other intrinsic property of elementary particles is the electric charge. Spin provides a measure of the intrinsic angular momentum of any particle. In contrast to classical mechanics, where angular momentum is associated with the rotation of a large object, spin is an exclusively quantum phenomenon, which cannot be directly related to rotation in space. The intuition that the spin corresponds to the angular momentum due to the rotation of the particle about its own axis should only be taken as a useful mental image, since, as deduced from the quantum theory relativistic, the spin does not have a representation in terms of spatial coordinates, so that no type of movement can be referred to. This implies that any observer when making a measurement of angular momentum will inevitably detect that the particle has a total intrinsic angular momentum, different observers differing only about the direction of said momentum, and not about its value (this last fact has no analogue in classical mechanics).).
Levels of organization of matter
Subatomic Particles
A subatomic particle is one that is smaller than an atom. It can be an elementary particle or one composed, in turn, by other subatomic particles.
The elementary particles are the elementary constituents of matter; more precisely they are particles that are not made up of smaller particles nor are they known to have internal structure.
In particle physics, fermions are particles that obey the Fermi‑Dirac statistics. Fermions can be elemental, like the electron, or compound, like the proton and neutron. In the Standard Model there are two types of elementary fermions: leptons and quarks, which are discussed below.
These quarks and leptons interact through four fundamental interactions: gravity, electromagnetism, weak interactions, and strong interactions. The Standard Model is currently the best explanation of all of physics, but despite decades of effort, gravity still cannot be considered at the quantum level; it is only described by classical physics (see quantum gravity and graviton). Interactions between quarks and leptons are the result of an exchange of force-carrying particles (such as photons) between quarks and leptons. Particles that carry force are not building blocks of matter. Consequently, mass and energy cannot always be related to matter. For example, the carriers of the electric force (photons) possess energy (according to Planck's constant) and the carriers of the weak force (the W and Z bosons) are massive, but neither is considered matter either. However, although these particles are not considered matter, they do contribute to the total mass of atoms or subatomic particles.
Atoms
An atom is the fundamental structural unit of matter that has the properties of a chemical element. A chemical substance is a particular class of homogeneous matter made up of atoms, either free or bound together, in defined proportions.
The fundamental structure of an atom is made up of a baryonic nucleus of protons and neutrons, and an orbital cloud of electrons attracted by the electromagnetic force.
Overview of the atomic nucleus
The protons and neutrons in the nucleus are attracted to each other by a different force, the nuclear force, which is generally stronger than the electromagnetic force that repels positively charged protons from each other. Under certain circumstances, more accentuated the more protons the atom has, the repelling electromagnetic force becomes stronger than the nuclear force and nucleons can be ejected or discarded from the nucleus, leaving behind a different element: nuclear decay that results in nuclear transmutation.
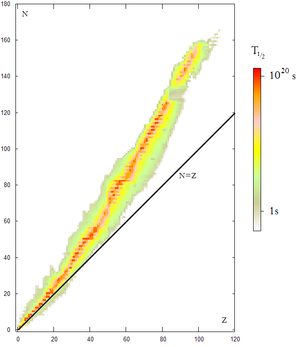
The number of protons in the nucleus defines which chemical element the atom belongs to: for example, all copper atoms contain 29 protons. The number of neutrons defines the isotope of the element.
Atomic nuclei have some kind of internal structure, for example neutrons and protons appear to be orbiting around each other, a fact that manifests itself in the existence of the nuclear magnetic moment. However, experiments reveal that the nucleus closely resembles a sphere or compact ellipsoid of 10−15 m (= 1 fm), in which the density seems practically constant. Naturally the radius varies according to the number of protons and neutrons, being the heavier nuclei and with more particles somewhat larger.
Atomic nuclei behave like composite particles at sufficiently low energies. Likewise, most atomic nuclei below a certain atomic weight and which also present a balance between the number of neutrons and the number of protons (atomic number) are stable. However, we know that isolated neutrons and nuclei with too many neutrons (or too many protons) are unstable or radioactive.
The explanation of this stability of the nuclei resides in the existence of pions. In isolation, neutrons can undergo, via a weak interaction, the following disintegration:
(1)n0→ → p++e− − +.. ! ! e{displaystyle n^{0}to p^{+}+e^{-}+{bar {nu }}{e}}
However, within the atomic nucleus, the proximity between neutrons and protons makes the reactions much faster, via strong interaction:
(2){n0▪ ▪ p++π π − − p+▪ ▪ n0+π π +{displaystyle {begin{cases}n^{0}rightleftarrows p^{+}pi ^{-}p^{+}rightleftarrows n^{0}+pi ^{+}end{cases}}}}
General information about the electronic cloud
An electron shell, electron shell, or electron shell designates the distribution of an orbital around the nucleus of an atom. Each shell can hold a certain maximum number of electrons and is associated with a particular energy range based on its distance from the nucleus. In a stable atom, for a certain shell to be able to contain electrons, it is necessary that all the shells inside it are completely occupied. The electrons in the outermost populated shell, called the valence shell and which is the only one that can be partially empty, determine the chemical properties of the atom.
Schrödinger's atomic model originally conceived of electrons as waves of matter. Thus the equation was interpreted as the wave equation that described the evolution in time and space of said material wave. Later Max Born proposed a probabilistic interpretation of the wave function of electrons. This new interpretation is compatible with electrons conceived as quasi-point particles whose probability of presence in a certain region is given by the integral of the square of the wave function in a region. That is, in the subsequent interpretation of the model, this was a probabilistic model that allowed empirical predictions to be made, but in which the position and the amount of movement cannot be known simultaneously, due to the uncertainty principle. Likewise, the result of certain measurements is not determined by the model, but only the set of possible results and their probability distribution.
An atomic orbital is the region of space defined by a particular spatial and time-independent solution to the Schrödinger equation for the case of an electron subjected to a Coulomb potential. The choice of three quantum numbers in the general solution points univocally to a possible one-electron state.
These three quantum numbers refer to the total energy of the electron, the orbital angular momentum and its projection on the z-axis of the laboratory system and are denoted by
r→ → 日本語nlm =END END n,lm(r→ → ){displaystyle langle {vec {r}{r}{rangle =psi _{n,l}^{m}({vec {r}}}}}}}
An orbital can also represent the time-independent position of an electron in a molecule, in which case it is called a molecular orbital.
The combination of all the atomic orbitals gives rise to the electronic shell, represented by the shell model, which adjusts to each chemical element according to the corresponding electronic configuration. For simplicity, the shapes of the angular part of the orbitals are collected, ignoring the radial nodes, which always have a spherical shape.
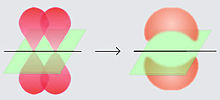
The s orbital has spherical symmetry around the atomic nucleus. The following figure shows two alternative ways to represent the electron cloud of an s orbital: in the first, the probability of finding the electron (represented by the density of points) decreases as we move away from the center; in the second, the spherical volume in which the electron spends most of the time is represented and lastly the electron is observed.
The geometric shape of the p orbitals is that of two spheres flattened towards the point of contact (the atomic nucleus) and oriented according to the coordinate axes. Depending on the values that the third quantum number ml (‑1, 0 and 1) can take, the three symmetric p orbitals are obtained with respect to the axes X, Z and y. Analogously to the previous case, the p orbitals have n‑2 radial nodes in the electron density, such that as the value of the principal quantum number increases, the probability of finding the electron moves away from the atomic nucleus. The "p" It also represents the energy that an electron possesses and increases as the distance between the nucleus and the orbital moves away.
The d orbitals have more diverse orientations. Four of them are in the form of 4 lobes of alternate signs (two nodal planes, in different orientations in space), and the last one is a double lobe surrounded by a ring (a double nodal cone). Following the same trend, they present n‑3 radial nodes. This has 5 orbitals and corresponds to the quantum number l (azimuthal)
The Pauli equation, or Schrödinger‑Pauli equation, is a generalization or reformulation of the Schrödinger equation for particles of spin 1/2 that takes into account the interaction between the spin and the electromagnetic field. This equation is the nonrelativistic limit of the Dirac equation and can be used to describe electrons for which relativistic velocity effects can be neglected. In general, a screening effect is one capable of attenuating a force or interaction. In atomic physics, the screening effect on the outermost electrons of an atom is described as the attenuation of the net attractive force on the electron, due to the presence of other electrons in lower shells and of the same energy level. The screening effect is a barrier of electrons of the same level, which exert repulsive forces on higher-level electrons, thus decreasing the probability of finding these electrons at lower levels. Each level produces screen tail effect; the greater the number of electrons, the greater the screening effect.
Within quantum physics this effect is the interference that exists between the last orbit of an atom and its nucleus.
The so-called Dirac equation is the relativistic version of the quantum mechanical wave equation and was formulated by Paul Dirac in 1928. It gives a description of elementary particles with spin ½, such as electron, and is completely consistent with the principles of quantum mechanics and the theory of special relativity. In addition to accounting for spin, the equation predicts the existence of antimatter.
Electronic configuration
The electronic configuration indicates the way in which electrons are structured, communicate or organize in an atom according to the model of electron shells, in which the wave functions of the system are expressed as an atom or atomically a product of antisymmetrized orbitals. Electronic configuration is important as it determines the chemical combination properties of atoms and therefore their position in the periodic table of elements.
According to quantum mechanics, electrons can pass from one atomic orbital to another either by emitting or absorbing a quantum of energy, in the form of a photon. This transition from one orbital to another with different energies explains various phenomena of emission and absorption of electromagnetic radiation by atoms.
The noble gases are a group of chemical elements with very similar properties: for example, under normal conditions, they are odorless, colorless monatomic gases and have very low chemical reactivity. They are located in group 18 (VIIIA) of the periodic table (previously called group 0).
The properties of noble gases can be explained by modern theories of atomic structure: their electron shell of valent electrons is considered complete,
The ionization energy or ionization potential (Ei) is the energy needed to separate a electron in its ground state of an atom of an element in a gaseous state. The reaction can be expressed as follows:
- A(g)+Ei→ → A+(g)+e− − {displaystyle mathrm {A(g)} +E_{rm {i}}to mathrm {A^{+}(g) +e^{-}} }.
In this case, a positively charged monatomic ion (monatomic cation) is formed.
The electron affinity (Eea) is defined as the energy released when a neutral gaseous atom in its ground state (in its lowest energy level) captures an electron and forms a mononegative ion:
- X(g)+e− − Δ Δ X− − (g)+Eea{displaystyle mathrm {X(g)+e^{-}} longrightarrow mathrm {X^{-}(g)}} +E_{rm {ea},!}
Since it is about released energy, since normally when inserting an electron in an atom the attractive force of the nucleus predominates, it has a negative sign. In cases where the energy is absorbed, when the repulsive forces win, they will have a positive sign.
We can also resort to the opposite process to determine the first electronic affinity, since it would be the energy consumed in extracting an electron from the mononegative anionic species in the gaseous state of a certain element; evidently the corresponding enthalpy Eea has a negative sign, except for noble gases and alkaline earth metals. This process is equivalent to that of the ionization energy of an atom, so the Eea would be, by this formalism, the ionization energy of zero order.
This property helps us to predict which elements will easily generate stable anionic monatomic species.
Atomic Bonds
A chemical bond is the chemical process responsible for attractive interactions between atoms and molecules, and which confers stability to diatomic and polyatomic chemical compounds. The explanation of such attractive forces is a complex area that is described by the laws of quantum chemistry.
An ionic bond or electrovalent bond is the result of the presence of electrostatic attraction between ions of different signs, that is, an anion-cation pair. The attraction Electrostatics between the oppositely charged ions cause them to bond. For an ionic bond to be generated, the difference (delta) of electronegativities must be greater than 1.7 or equal. (Pauling scale; according to the Van Arkel-Ketelaar Triangle).
It should be noted that no bond is fully ionic, there will always be a contribution in the bond that can be attributed to the sharing of electrons in the same bond (covalence). The ionic bond model is a convenient exaggeration since many thermodynamic data can be obtained with very good precision if the atoms are thought of as ions and there is no sharing of electrons.
A covalent bond is implicit in the Lewis structure indicating shared electrons between the atoms. A covalent bond between two atoms occurs when these atoms unite, to reach the stable octet, sharing electrons of the last level (except hydrogen which reaches stability when it has 2 electrons). The difference in electronegativity between the atoms is not large enough for an ionic bond to occur. For a covalent bond to be generated, it is necessary that the difference in electronegativity between atoms is less than 1.7.
In chemistry, the valence bond theory (TEV) explains the nature of a chemical bond in a molecule, in terms of atomic valences. The valence bond theory is summarized in the rule that the central atom in a molecule tends to form pairs of electrons, in accordance with geometric constraints, as defined by the octet rule. The valence bond theory is closely related to the theory of molecular orbitals.
An important aspect of valence bond theory is the condition of maximum overlap that leads to the formation of the strongest possible bonds. This theory is used to explain the formation of covalent bonds in many molecules.
For example, in the case of the molecule F2, the F‑F bond is formed by the overlapping p orbitals of two different fluorine atoms, each containing an unpaired electron. Since the nature of the orbitals is different in H2 and F2 molecules, the bond strength and bond length will differ in both molecules.
In an HF molecule, the covalent bond is formed by the overlap of the 1s orbital of H and the 2p orbital of F, each containing an unpaired electron. The mutual sharing of electrons between H and F results in the formation of a covalent bond between them.
In chemistry, hybridization is the interaction of atomic orbitals within an atom to form new hybrid orbitals. Hybrid atomic orbitals are those that overlap in the formation of bonds, within the valence bond theory, and justify molecular geometry.
The sigma bond (σ bond) is the strongest type of covalent chemical bond, even stronger than the pi bond, which forms the double bond. The sigma orbital is more clearly defined for diatomic molecules using the language and tools of group symmetry.
pi bonds (π bonds) are covalent chemical bonds where two lobes of one orbital involved in the bond overlap with two lobes of the other orbital involved. These orbitals share a nodal plane that passes through the nuclei involved.
The coordination or coordinate bond, also known as a dative covalent bond or bipolar bond, is a covalent bond in which each pair of electrons shared by two atoms is contributed by one of them. The atom that contributes the pair of electrons is called the donor, and the one that receives it, the receiver.
The molecular orbital theory (TOM) is a method of determining chemical bonding in which electrons are not assigned to individual bonds between atoms, but rather they move under the influence of the nuclei of the whole molecule.
Molecular orbitals are regions of space that contain the electron density defined by mathematical functions that describe the wave behavior that electrons can have in molecules. These functions can be used to calculate chemical and physical properties such as the probability of finding an electron in a region of space. The term orbital was first introduced in English by Robert S. Mulliken in 1932 as an abbreviation for "one-electron orbital wave function".) from a translation of the German word used in 1925 by Erwin Schrödinger, 'Eigenfunktion'. Since then it is considered a synonym of the region of space generated with this function. Molecular orbitals are usually built by linear combination of atomic orbitals centered on each atom in the molecule. Using electronic structure calculation methods, such as the Hartree-Fock method or the self-consistent field (self-consistent field, SCF), they can be obtained quantitatively.
The crystal field theory (TCC) is a theoretical model that describes the electronic structure of those transition metal compounds that can be considered coordination compounds. Crystal field theory successfully explains some of the magnetic properties, colors, hydration enthalpies, and spinel (octahedral) structures of transition metal complexes, but fails to describe the causes of bonding. CBT was developed by the physicists Hans Bethe and John Hasbrouck van Vleck in the 1930s. CBT was later combined with molecular orbital theory to produce the ligand field theory, which is slightly more complex but also more complex. adjusted to reality, since it also delves into the explanation of the process of formation of the chemical bond in metal complexes.
A metallic bond is a chemical bond that holds together the atoms (union between atomic nuclei and valence electrons, which gather around them like a cloud) of metals.
These atoms pack very closely together, producing very compact structures. These are three-dimensional lines that acquire structures such as: the typical compact packing of spheres (compact hexagonal), cubic centered on the faces or the cubic centered on the body.
Molecular Theory
Overview of Molecules
A molecule is an electrically neutral and sufficiently stable group of at least two atoms in a defined configuration, held together by strong chemical bonds (covalent or ionic bonds).
In this strict sense, molecules differ from polyatomic ions. Molecular geometry refers to the three-dimensional arrangement of the atoms that make up a molecule. It determines many of the properties of molecules, reactivity, polarity, phase, color, magnetism, biological activity, etc. Currently, the main model is the valence electron pair repulsion theory (VTRPE), used internationally for its great predictability.
Molecular symmetry describes the symmetry of molecules and uses this criterion for their classification. Molecular symmetry is a fundamental concept in chemistry, since many of the chemical properties of a molecule, such as its dipole moment and the allowed spectroscopic transitions (based on selection rules such as Laporte's rule) can be predicted or explained from the symmetry of the molecule. Although there are several theoretical frameworks in which molecular symmetry can be studied, group theory is the main one. Many techniques exist to empirically establish molecular symmetry, including X-ray crystallography and various forms of spectroscopy.
Molecular topology is a part of mathematical chemistry and deals with the algebraic description of chemical compounds, allowing a unique and easy characterization of them. The topology is not sensitive to the details of a scalar field, and can often be determined by simplified calculations. Scalar fields such as electron density, Madelung field, covalent field, and electrostatic potential can be used to establish the topology model.
A macromolecule is a large molecule commonly created through the polymerization of smaller subunits (monomers). They are usually made up of thousands, or more, of atoms. They can be both organic and inorganic and the most common in biochemistry are biopolymers (nucleic acids, proteins, carbohydrates and polyphenols) and large non-polymeric molecules (such as lipids and macrocycles). Synthetic macromolecules are common plastics and synthetic fibers, as well as some experimental materials such as carbon nanotubes.
Intermolecular Interactions
In quantum mechanics, under the probabilistic interpretation, the particles cannot be considered punctual, but are spatially delocalized before making a measurement on their position. The electron density is a distribution that determines the spatial probability of one or more identical particles.
Polarizability is the relative tendency of a charge distribution, such as the electron cloud of an atom or molecule, to be distorted from its normal shape by an external electric field, which can be caused by by the presence of a nearby ion or a dipole. Electronic polarization is a displacement of charges in the presence of an external electric field, that is, in a neutral atom the electronic cloud is reoriented in such a way that the atom is slightly distorted and loses its symmetry. The difficulty in analyzing these phenomena varies in the treatment of many-body interaction.
Electronic polarization α α {displaystyle alpha } is defined as the reason of the induced dipolar moment p{displaystyle p} of an atom to the electric field E{displaystyle E} which produces such a dipolar moment. One Intermolecular force refers to the interactions that exist between molecules according to their nature. Generally, the classification is made according to the polarity of the molecules that are interacting, or on the basis of the nature of the molecules, of the elements that make up it.
The electronegativity is the ability of an atom to attract electron density, when it forms a chemical bond in a molecule. We must also consider the distribution of electron density around of a certain atom compared to other different ones, both in a molecular species and in non-molecular systems or species.
When a molecule is formed in a covalent bond mode, the pair of electrons tends to move towards the atom with the greater electronegativity. This causes an asymmetric electronic density between the nuclei that form the bond, which is called a polar covalent bond (an electric dipole is formed). The bond is more polar the greater the difference between the electronegativities of the atoms that are bonded. Chemical polarity is a property of molecules derived from the vector sum of the dipole moments of the polar covalent bonds in a molecule. This property is closely related to other properties such as solubility, melting point, boiling point, intermolecular forces, etc.
Weak, non-covalent interactions are called "weak" because they represent the energy that holds molecular species together and are considerably weaker than covalent bonds. The fundamental non-covalent interactions are:
- Hydrogen Bridge Force
- The forces of Van der Waals, which we can in turn classify in:
- ion-dipolo.
- dipolo ‐ dipolo.
- dipolo ‐ induced dipolo.
- London dispersal forces known as dispersion forces, London forces or induced instant dipolo-dipole forces.
Kinetic Molecular Theory
The kinetic theory of gases is a physical and chemical theory that explains the behavior and macroscopic properties of gases (ideal gas law), based on a statistical description of molecular processes microscopic.
This branch of physics describes the thermal properties of gases. These systems contain enormous numbers of atoms or molecules, and the only reasonable way to understand their thermal properties on the basis of molecular mechanics is to find certain average dynamical quantities and relate the observed physical properties of the system to these averaged molecular dynamical properties.. The techniques to relate the global macroscopic behavior of material systems with the average behavior of their molecular components constitute statistical mechanics.
- The number of molecules is large and the average separation between them is large compared to their dimensions. Therefore, they occupy a despicable volume compared to the volume of the container and are considered to be punctual masses.
- The molecules obey Newton's laws, but individually move randomly, with different speeds each, but with an average speed that doesn't change over time.
- The molecules perform elastic shocks among themselves, therefore the linear moment and the kinetic energy of the molecules are preserved.
- The forces between molecules are despicable, except during shock. Electrical or nuclear forces among molecules are considered to be short-range, so only the impulsive forces that arise during shock are considered.
- Gas is considered pure, i.e. all molecules are identical.
- The gas is in thermal balance with the walls of the container.
- La Law of Graham establishes that the speeds of diffusion and diffusion of gases are inversely proportional to the square roots of their respective molar masses:
v1v2=M2M1{displaystyle {{mbox{v}}_{1} over {mbox{v}_{2}}}}={sqrt {M_{2} over M_{1}}}}}}}}}}}}}
Being v{displaystyle v} speeds and M{displaystyle M} the molar masses.
Effusion is the flow of gas particles through narrow openings or pores. Use is made of this principle in the effusion method of isotope separation. The effusion phenomenon is related to the kinetic energy of the molecules. Thanks to their constant motion, the particles of a substance are evenly distributed in free space. If there is a greater agglomeration of particles at a point, there will be more collisions with each other, which will cause them to move towards the regions with fewer numbers: substances diffuse from a region of greater agglomeration to a region of lesser agglomeration.
A real gas, as opposed to an ideal or perfect gas, is a gas that exhibits properties that cannot be fully explained using the ideal gas law. To understand the behavior of real gases, the following must be taken into account:
- ‐ compressibility effects
- ‐ variable specific heat capacity
- forces of Van der Waals
- thermodynamic effects of non-balance
- ‐ issues with molecular dissociation and elemental reactions with variable composition.
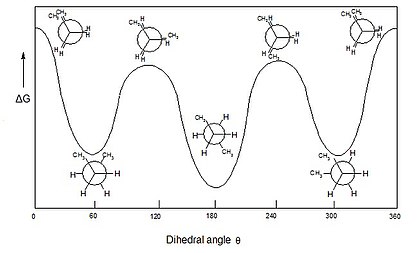
Conformational analysis is the exploration of all the conformers that can be obtained of a given molecule by performing twists around single bonds (conformational degrees of freedom), observing the changes in molecular energy associated with those twists.
The term macromolecule originally referred to molecules that weighed more than 10,000 daltons in atomic mass, although they can reach millions of AMUs.
Infrared spectroscopy exploits the fact that molecules absorb frequencies that are characteristic of their structure. These absorptions occur at resonant frequencies, that is, the frequency of the absorbed radiation coincides with the frequency of vibration. The energies are affected by the shape of the molecular potential energy surfaces, the masses of the atoms, and the associated vibronic coupling. In particular, in the Born-Oppenheimer and harmonic approximations, that is, when the molecular Hamiltonian corresponding to the electronic ground state can be approximated by a harmonic oscillator in the neighborhood of the equilibrium molecular geometry, the resonant frequencies are associated with the normal modes corresponding to the potential energy surface of the ground state of molecular electronics. Resonant frequencies are also related to the strength of the bond and the mass of the atoms at each end of the bond. Therefore, the frequency of the vibrations is associated with a particular normal motion mode and a particular link type.
1.- Classification of matter
Matter can be found in nature in the form of pure substances and mixtures.
The pure substances are those whose nature and composition do not vary whatever their state. They are divided into two large groups: Elements and Compounds.
Elements: They are pure substances that cannot be broken down into other simpler pure substances by any means. Example: All the elements of the periodic table: oxygen, iron, carbon, sodium, chlorine, copper, etc. They are represented by their chemical symbol and 115 are known in the actuality.vv
Physics of Condensed Matter
The physics of condensed matter is the field of physics that deals with the macroscopic physical characteristics of matter. In particular, it refers to the "condensed" phases that appear whenever the number of constituents in a system is extremely large and the interactions between the components are strong. The most familiar examples of condensed phases are solids and liquids, which arise from the bonds and junctions caused by electromagnetic interactions between atoms.
Solid bodies are made up of densely packed atoms with strong forces of interaction between them. Interaction effects are responsible for the mechanical, thermal, electrical, magnetic, and optical properties of solids.
Except for glass and amorphous substances, whose structure does not appear ordered but disorganized, all solid matter is in a crystalline state. In general, it occurs in the form of an aggregate of small crystals (or polycrystalline) as in ice, very hard rocks, bricks, concrete, plastics, highly proportional metals, bones, etc.
They can also form single crystals of minuscule dimensions such as sugar or salt, precious stones and most minerals, some of which are used in modern technology for their sophisticated applications, such as quartz in oscillators or semiconductors in electronic devices.
Solids can be classified according to the nature of the bond between their atomic or molecular components. The traditional classification distinguishes four types of link:
- Covalent link, forming solid covalent network (sometimes simply called "covalently onlyds").
- Ionic link, which forms ionic solids.
- Metallic link, forming metallic solids.
- weak intermolecular link, forming molecular solids.
Bose‑Einstein Condensate
This state of matter was discovered by Satyendra Nath Bose, who sent his paper on photon statistics to Einstein for comment. Following the publication of the Bose paper, Einstein extended his treatment to a number of fixed particles (atoms) and predicted this fifth state of matter in 1925. Bose-Einstein condensates were first made experimentally by several different groups in 1995. for rubidium, sodium and lithium, using a combination of laser and evaporative cooling . Bose-Einstein condensation for atomic hydrogen was achieved in 1998 . The Bose-Einstein condensate is a superfluid-like liquid that occurs at low temperatures in which all atoms occupy the same quantum state. In low-density systems, occurring at or below 10−5 K .
Supramolecular Systems
supramolecular chemistry is the branch of chemistry that studies supramolecular interactions, that is, between molecules. His study is inspired by biology and is based on the mechanisms of synthetic organic and inorganic chemistry.
Supramolecular chemistry studies molecular recognition and the formation of supramolecular aggregates, which gives us the opportunity to understand and interface the biological world, complex systems and nanotechnology. Supramolecular chemistry is defined as:
"Supramolecular chemistry is the chemistry of intermolecular bonds, covering the structures and functions of entities formed by the association of two or more chemical species" J‑M‑Lehn
"Supramolecular chemistry is defined as chemistry beyond the molecular, a chemistry of designed intermolecular interactions" F. Vögtle
The supramolecular aggregates that are the object of study by supramolecular chemistry are very diverse, ranging from biological systems involving a large number of molecules that spontaneously organize themselves to form larger structures, such as monolayers, bilayers, micelles, complexes enzymes and lipoproteins, to assemblies of few molecules that undergo a phenomenon of molecular self-assembly, such as catenanes, rotaxanes, molecular polyhedra and other related architectures.
Solvation is the process of formation of attractive interactions between molecules of a solvent with molecules or ions of a solute. In solution, ions of the solute disperse and are surrounded by molecules of solvent, the same happens in the solvent molecules.
The cryptands are a family of synthetic bi‑ and polycyclic multidentate ligands that possess affinity for a variety of cations. The 1987 Nobel Prize in Chemistry was awarded to Donald J. Cram, Jean‑ Marie Lehn—who first studied them in 1969—and Charles J. Pedersen for their work in discovering and determining the uses of cryptands and crown ethers, beginning the field of supramolecular chemistry. The term cryptand implies that the ligand retains substrates in a crypt, confining the guest as in a burial. These molecules are the three-dimensional analogues of crown ethers but are more selective and trap ions with greater forces. The resulting complexes are lipophilic.
A clathrate, clathrate structure or clathrate compound (from Latin clathratus, " surrounded or protected, lattice") is a chemical substance formed by a network of a certain type of molecule, which traps and retains another type of molecule.
A gas hydrate is, for example, a special type of clathrate in which the water molecule forms a structure capable of containing a gas. A clathrate is a supramolecular inclusion system in which molecules of the appropriate size (2-9 Angstrom) are trapped in cavities that appear in the structure of another compound.
Surface phenomena
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid-liquid, solid-gas, solid-vacuum, liquid-gas interfaces. It is an interdisciplinary science with overlapping fields of surface chemistry and surface physics. As a science it is a subfield of materials science. Surface physics studies the physical changes that occur at interfaces. Some of the aspects that this branch of physics studies include superficial reconstructions; the electronic plasmon and acoustic transitions at phonon surfaces; the epitaxy; electronic issuance; electronic tunneling; the assembly of surfaces or the formation of nanostructures.
Adsorption is a process by which atoms, ions or molecules of gases, liquids or dissolved solids are trapped or retained on a surface, as opposed to absorption, which is a phenomenon of volume. That is, adsorption is a process in which, for example, a soluble contaminant (adsorbate) is removed from water by contact with a solid surface (adsorbent). The reverse process to adsorption is known as desorption.
Nucleation can refer to different disciplines and is a key process to understand the thermal processing of polymers, alloys and some ceramics. In chemistry and biophysics, nucleation can refer to the formation of multimers, which are intermediates in polymerization processes. This type of process is believed to be the best model for processes such as crystallization and amyloidogenesis.
In physics and chemistry, a colloid, colloidal system, colloidal suspension or colloidal dispersion is a system made up of two or more phases, normally one fluid (liquid) and the other dispersed in the form of very fine solid particles, with a diameter between 10−9 and 10−5 m. The dispersed phase is the one found in the smallest proportion. Normally the continuous phase is liquid, but colloids can be found whose components are in other states of aggregation of matter.
Properties of ordinary matter
General Properties
The general properties present the basic material systems without distinction and therefore do not allow differentiating one substance from another. Some of the general properties are called extensive, since their value depends on the amount of matter, such is the case of mass, weight, volume. Others, those that do not depend on the amount of matter but on the substance in question, are called intensive. The paradigmatic example of intensive magnitude of mass matter is density.
Extrinsic or general properties
They are the qualities that allow us to recognize matter, such as extension, or inertia. They are additive because they depend on the amount of the sample taken. To measure them we define magnitudes, such as mass, to measure inertia, and volume, to measure extension (it is not really an exact additive property of matter in general, but for each substance in particular, because if we mix, for example, 50 ml of water with 50 ml of ethanol, we obtain a solution volume of 96 ml). There are other general properties, such as interaction, which is measured by strength. Every material system interacts with others in a gravitational, electromagnetic or nuclear way. Its corpuscular structure is also a general property of matter, which justifies that the quantity is measured for certain uses in moles.
Intrinsic or specific properties
They are the qualities of matter independent of the quantity in question, that is, they do not depend on mass. They are not additive and generally result from the composition of two extensive properties. The perfect example is provided by density, which relates mass to volume. It is also the case of the melting point, the boiling point, the solubility coefficient, the refractive index, the Young's modulus, etc.
Chemical properties of matter
They are those distinctive properties of substances that are observed when they react, that is, when chemical bonds are broken or formed between atoms, forming new substances different from the original ones with the same matter. Chemical properties are manifested in chemical processes (chemical reactions), while the proper physical properties are manifested in physical processes, such as change of state, deformation, displacement, etc.
Examples of chemical properties:
- Causticity of the Base
- Corrosiveness of acids
- Heat power or caloric energy
- Acidity
- Alkalinity
- Reactivity
Miscellaneous
- The kilogram is a unit of the amount of matter, corresponds to the mass of a dm3 (1 liter) of pure water to 4 °C of temperature. From this measure, a platinum block and iridium of the same mass was created which was named kilogram pattern. This is preserved in the International Office of Weights and Measures of Sèvres (France).
- The amount of matter can also be estimated by the energy contained in a certain region of space, as suggested by the formula E = m·c2 that gives the equivalence between mass and energy established by the theory of relativity of Albert Einstein.
- "Densities size" in [kg/m3]: osmio 22300, gold 19300, iron 7960, cement 3000, water 1000, ice 920, wood 600 to 900, air 1, 29.
- Temperature is a magnitude that indicates the degree of thermal agitation of a substance. Also, when two substances in contact have different temperatures, a thermal energy transfer (in the form of heat) occurs until the temperatures are equal. At the time temperatures are equal, it is said that these two substances are in thermal balance.
- The three most abundant chemical elements in the universe are H, He and C; some of its most important properties are:
- Hydrogen (H)2): density = 0, 0899 kg/m3 Teb = ‐252, 9 °C, Tf =-259, 1 °C.
- Helio (Hel): density = 0, 179 kg/m3 Teb = ‐268, 9 °C, Tf = ‐272, 2 °C.
- Carbon (C): density = 2267 kg/m3 Teb = 4027 °C, Tf = 3527 °C.
77Bibliography
- Landau & Lifshitz, Classic Theory of the FieldsEd. I reversed, ISBN 84-291-4082-4.
- Vázquez‐Reyna Mario. Reflections on matter, energy and mass. Mexico City (1998). ISBN 970-91797-1-3.
- Segura González, Wenceslao, Relativistic Field Theory, eWT Editions, 2014, ISBN 978-84-617-1463-6.
Contenido relacionado
Mitochondrial crest
Chlorofluorocarbon
Kilogram per cubic meter