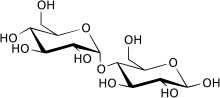
Maltase
The maltase is an enzyme that catalyzes the hydrolysis of maltose (disaccharide) into the two simple glucoses that compose it. In humans it is present in the small intestine and plays a key role in digestion. It belongs to the family of disaccharidases, enzymes responsible for hydrolyzing disaccharides into the monosaccharides that form them.
Starch digestion requires six intestinal enzymes, two of which are α-amylases. The remaining four have been identified as maltases, exoglycosidases attached to the luminal surface of enterocytes.
In most cases it is equivalent to saying alpha-gluosidase to maltase, although the term "maltase" emphasizes the disaccharide nature of the substrate from which glucose hides, while "alpha-glucosidase" emphasizes in the link regardless of the size of the carbohydrate.
Nomenclature and classification
Maltase belongs to the family of α-glucosidases (essential for breaking down glycogen into glucose), and is found in the brush border of the intestinal microvilli of the small intestine. Since maltase catalyzes the digestion of linear starch oligosaccharides (without α(1→6) branches) to glucose, they are also known as α-glucosidases; although there are also certain maltases such as sucrase-isomaltase that are capable of breaking these α(1→6) bonds to make the molecules more linear and allow the degradation of maltases-glucoamylases.
Maltase-glucoamylase
Plays a crucial role in the digestion of starch. Thanks to this enzyme, human beings are able to digest starches of vegetable origin, since it degrades the α(1→4) glycosidic bonds, which are the most linear, and in this way, glucose units are obtained from large polysaccharides. they are vital in human metabolism.
Sucrose-glucoamylase
It is essential for the digestion of carbohydrates, including starch, sucrose, and isomaltose. It works together with maltase-glucoamylase to degrade large polysaccharides, specifically it is responsible for catalyzing the hydrolysis of α(1→6) bonds that may exist.
Alpha-amylase 1
Its function is to cleave the α-glucosidase bonds in the oligosaccharides and polysaccharides that make up the compound so that the rest of the enzymes can catalyze other reactions. Just as the other enzymes act on disaccharides or oligosaccharides, in the first place it is vital to split the macromolecules into smaller molecules that can be degraded by the other enzymes.
Structure
Maltase or α-glucosidase belongs to the GH13 family of intestinal enzymes that act on substrates with alpha-glycosidic bonds. The following genes can code for maltase:
- Maltase-glucoamilasa (codified in humans by the MGAM gene) is a digestive enzyme composed of a cytolic domain, a transmembrane domain, an O-glycosydic link and two enzyme units:
- Extrem N-terminal: composed of five proteic domains (a first domain of cysteine-rich clover P, a second, fourth and fifth domain formed by two antiparalelate beta sheets and the third domain that is the largest and the one that gives it catalytic activity). To this end N-terminal cannot be coupled too large substrates and therefore proves affinity to the maltosa, maltotriosa, maltotetrosa and maltotheentosa.
- Extrem C-terminal: contains additional binding sites and therefore can degrade larger substrates and has about 21 amino acids more than the N-terminal end (explaining its wider function).
- The α-glucosidase acid, encoded by the GAA gene and whose main function is the lysis of complex sugars. This acid contains approximately 952 amino acids and is processed post-translationally by glycosylation and removal of peptides at the end N- and C- terminals.
- The sacral-isomaltase (codified by the SI gene) is an enzyme complex that is found on the margins of the small intestine's hair. It is made up of 1827 amino acids and contains more than 19 sites of glycosylation. It has a cytolic domain, a transmembrane domain, an O-glycosydic link and two homologous subunits, the sacrosanct and the isomaltasa, 120 kDa and 140 kDa respectively. The isomaltase subunit is responsible for the anchoring of the enzyme with intestinal cells as it has a hydrophobic segment of amino acids specialized in the association with the membrane of these cells.
- The α-amilasa is an enzyme (gen AMY1A) with a molecular weight of 50kDa and three structural domains:
- The domain A, also known as the central domain, is formed by 8 parallel β-filled sheets surrounded by 8 propellers α.
- The smaller B domain is between the third propeller α and the β sheet that precedes it.
- The C domain, whose function is not yet well defined, is attached to the A domain. It has an anti-parallel β structure.
- In specific organisms, there are also additional domains (C, D, F, G, H and I) attached to domain A. Your function is not yet well defined.
Summary
In mammals
Mammalian intestinal microvilli maltase is synthesized as a single polypeptide chain that does not undergo any type of proteolytic cleavage, neither intracellular nor extracellular. Mannose-rich carbohydrates are added to this by cotranslational glycosylation in those membrane domains where Ca2+ precipitates.
In yeasts
Yeast present, at five different loci, the group of MAL genes (MAL1, MAL2, MAL3, MAL4 and MAL6). The presence of at least one of these genes is essential for maltase synthesis to take place, since it suggests that MAL genes code for proteins that regulate this process. Consequently, the deficiency of these genes in yeasts would imply their inability to ferment maltose.
Gen
The gene that codes for maltase is located on chromosome 7 of the karyotype and has the following amino acid sequence:
M-D-L-H-L-H-L-P-A-S-S-S-T-V-S-I-L-T-S-F-T-N-K-S-A-M-I-V-Y-Y-C-V-I-F-F-D-L-F-S-S-R-G-I-S-I-K-L-P-E-N-P-N-P-Q-P-L-K-W-W-Q-S-E-I-I-Y-Q-V-Y-P-R-S-F-M-D-S-D-G-D-G-V-G-D-L-K-G-I-E-S-K-V-D-Y-I-A-D-L-G-A-G-A-V-W-L-S-P-I-Y-E-S-P-M-K-D-F-G-Y-D-I-S-N-F-T-N-I-D-P-I-F-G-T-M-D-D-F-L-S-L-A-T-K-L-R-E-N-G-L-K-L-V-M-D-F-V-P-N-H-S-S-D-L-H-V-W-F-N-L-S-V-H-R-Q-E-K-Y-E-D-F-Y-V-W-K-N-A-S-G-Y-D-S-E-E-N-P-I-V-P-N-N-W-V-S-F-F-G-G-S-A-W-E-W-N-E-I-R-E-Q-F-Y-F-H-Q-F-V-A-G-Q-P-D-L-N-F-E-N-P-E-V-L-H-E-M-L-E-I-M-K-F-W-L-D-M-G-V-D-G-F-R-M-D-T-V-P-S-M-F-E-D-Q-R-F-L-D-E-A-E-D-P-N-R-P-P-N-S-I-P-T-D-Y-S-Y-W-S-H-I-Y-T-Y-D-L-P-A-T-R-D-S-W-Q-I-S-E-N-Y-L-I-S-D-G-K-E-R-C-M-M-I-E-A-Y-V-G-S-L-D-K-L-F-L-Y-Y-G-T-K-T-K-PI-A-H-F-P-E-N-F-E-F-I-T-R-S-N-Q-V-D-F-N-M-L-I-L-L-L-P-G-S-A-V-T-Y-Y-G-E-E-I-G-M-T-N-T-N-L-T-W-E-E-T-V-D-P-P-G-C-Q-A-G-P-E-R-Y-Q-L-F-S-R-D-P-E-R-T-P-M-Q-W-N-Y-D-K-N-S-G-F-S-T-G-D-S-T-W-L-P-I-N-P-N-Y-K-E-L-N-V-Q-A-Q-N-ET-S-G-D-S-H-L-K-V-Y-K-Q-I-L-S-L-R-K-T-D-T-W-K-Y-G-SL-E-A-Y-A-L-E-N-G-Q-G-F-G-F-A-R-I-Y-K-G-S-G-F-I-V-L-A-N-Y-L-D-G-M-I-I-N-V-G-E-H-F-K-D-I-P-D-S-A-M-V-Y-T-T-S-V-N-F-Q-P-E-M-S-V-G-S-F-I-S-T-K-S-V-T-I-R-G-K-H-S-I-I-L-T-F-S-K-S-E-N-E-K-K-N-D-T-W-I-W-T-I-V-N-W-I-L-L-S-K-A-N-F-Y-L-F-T-I-I-S-D-L-N-N-G-H-I-V-K-P-V-W-N-L-Q-N-N-Y-N-T-V
This amino acid sequence is expressed with a letter code, and it should be noted that it can vary slightly in each species and each type of enzyme, as well as due to mutations that may or may not render the enzyme useless.
| Enzima | People who encode it |
|---|---|
| Alpha-glucosidase | GAA |
| Maltasa-glucoamilasa | MGAM |
| Sacarasa-isomaltasa | Yes |
| Alfa-amilasa 1 | AMY1A |
Two major α-glucosidase (maltase) genes, sucrase-isomaltase (SI) and maltase-glucoamylase (MGAM), respectively, are expressed in the small intestine.
The MGAM gene encodes a terminal starch glucosidase with high activity. This is inhibited by the oligomers produced by the hydrolysis of starch by α-amylase.
SI is a terminal starch glucosidase with low activity. This is inhibited by the oligomeric products of α-amylase. Its activity is greatly amplified by internal α-amylase activity.
Enzyme mechanism
Insufficiency of α-amylase in the human body is counteracted by the digestion of starch by mucosal maltase.
4 different activities of the duodenal mucosa are known to involve maltase. Two of these maltase activities are associated with sucrase-isomaltase and the remaining two are maltase-glucoamylases (maltases II and III) without distinctive features.
As it is an enzyme belonging to the GH13 family, its mechanism is based on the cleavage of the α-glucosidic bond by hydrolysis. Maltase focuses its activity on cleaving the α-(1→4) bond between the two glucose units that make up maltose. The hydrolysis rate is controlled by the substrate (number of carbohydrates).
Molecular regulation
It is believed that maltase is synthesized by cells of the mucous membrane lining the duodenal wall.
The flexibility of digestive enzymes in response to changes in the level of macronutrients in the diet is common in mammals and birds. It has been shown that in rodents a change of diet to one higher in carbohydrates causes an increase in the transcriptional control of intestinal SI and MGAM in a period of six to twelve hours. The same thing happened in house sparrow chicks. In turn, a reverse switch back to habitual diets causes a decrease in SI mRNA and its activity. However, this enzymatic flexibility is not universal as there are animals incapable of adapting in this way.
Recent information suggests that the signal caused by the existence of glucose in the duodenal mucosa is directly associated with the expression of the sucrase-isomaltase (SI) and maltase-glucoamylase (MGAM) genes through a histone code such as acetylation and dimethylations/trimethyls, and that the promoter structures of the MGAM and SI genes are similar. Regulations of these gene expressions are related to both the quantity and quality of carbohydrates fed. Therefore, a change in gene expression is accompanied by drastic changes in chromatin caused by histone modifications, and consequently directly influences the regulation of gene transcription.
It is known that histone H3 is responsible for driving the promoter and transcribed regions of SI and MGAM, and that histone H3 and H4 acetylations, as well as the general control of amino acid synthesis and production factors. Transcription of the transcribed regions of the SI and MGAM genes are increased in the presence of a starchy diet.
Modulations of MGAM and SI messages are mediated by histone acetylations and methylations of the promoter and transcribed regions. Likewise, lysine and arginine are post-translationally modified in relation to the different states of transcription induced by the presence of starches.
Fructose
Fructose actively modulates SI messages.
Starch
Starches modulate the expression of MGAM and SI messages.
These findings indicate that carbohydrate-mediated regulation of intestinal genes involves two mechanisms: one related to the amount of carbohydrate in the diet, and another specifically caused by fructose.
Tissue distribution
Both glucoamylase maltase (MGAM) and sucrose isomaltase (SI) act together on the brush border of the microvilli of the epithelium of the small intestine. The localization of these enzymes in these intestinal tissues is due to the continuous passage of nutrients and carbohydrates on which they can act and catalyze their cleavage through glycosidic bond hydrolysis.
Occurrence in animals
Macronutrients present in the diet of animals regulate the presence of maltase. Likewise, flying mammals have a lower presence of these enzymes in the small intestine since it has a greater permeability to hydrophilic organic solutes, such as glucose, which are passively absorbed. This explains the greater presence of microvilli and tight-junctions in both flying mammals and birds, and the worse adaptation of the mechanism of regulation of enzyme molecules to a change in diet.
It is also found in plants, bacteria, vertebrates, and yeast. The family Desmodontinae appears to be the only vertebrate that does not show intestinal maltase activity.
History
The history of the discovery of maltase is tied to the use of maltose. This dates back to 1806, when due to the "Berlin Decree" imposed by Napoleon Bonaparte caused a continental blockade of sugar to the invaded countries that led to the beginning of the search for alternative sugar sources. This led to the discovery of sugar beets as an alternative source of sugar and later the discovery of α -amylase as a starch digestion enzyme in the year 1833 by Payen.
In 1856, Claude Bernard discovered sucrase activity in the small intestine, then called invertase. He found this activity in a wide variety of mammals, including Homo sapiens sapiens. In the 20th century, Borkström's group in Sweden concluded that the luminal concentration of invertase was small compared to that of α -amylase.
In the 1880s the activity of intestinal maltase was discovered and distinguished from amylase G.T. Brown since amylase hydrolyzed starch to maltose, and maltase hydrolyzed maltose to glucose.
By the 1960s, advances in biochemistry enabled Dahlqvist and Semenza to fractionate and characterize 2 main peptide structures in which they found 4 intrinsic enzymes responsible for maltase activity. In addition, a maltase peptide was active in the hydrolysis of sucrose and isomaltase-derived substrates. The remaining peptide structure was named maltase-glucoamylase.
Thanks to current advances, biochemical observation and therefore understanding of these maltase fractions is possible. Between 1992 and 1998, thanks to the cloning and sequencing of mucosal starch hydrolase enzymes, it was confirmed that there are 2 maltase activities expressed by maltase-glucoamylase and two by sucrose-glucoamylase.
Current scientific activity is focused on detailing the structural specificity of this enzyme, the modulation of its activity, the selection of substrate and pathologies related to the lack or absence of maltase activity.
Industrial applications
Fermentation
The maltase enzyme plays a crucial role in fermentation, a process by which the yeast consumes the sugars that are present in the dough and that come from the degradation of the starch in the flour: glucose, sucrose, fructose and maltose. The last disaccharide to be consumed is maltose, and this is a problem since the bread-making process becomes slower and less efficient in terms of the physical qualities of the material.
For this reason, the Brist-Brocades company developed a method to solve the problem of late use of maltose. First, they confirmed that for yeast to be able to metabolize maltose, the enzymes maltose permease and maltase must be active. The first enzyme is responsible for using maltose from the medium and introducing it into the yeast, and the second, breaks down the maltose. When there are high glucose levels, a blockage occurs in the promoter regions, which are responsible for the gene expression of the genes that synthesize both enzymes, in the case of maltase, the GAA gene. This blockage was solved by genetically modifying the yeast to consume maltose first: the transgenic yeast known as MAL yeast. The result obtained was very positive: the production of carbon dioxide and the fermentative capacity were increased, therefore, in this way, the time spent in the process was reduced.
MAL yeast was the first transgenic yeast to obtain marketing permission.
In the near future, the need for this type of enzyme production on dairy products will increase significantly, due to the need for dairy products with high nutritional value in the country to overcome malnutrition and obesity and shift towards low-fat foods. and healthy.
Brewing industry
The first step in beer production is malting, where starches are transformed into maltose and dextrins. Maltose is a usable sugar for alcoholic fermentation, and dextrins are related to flavor.
The role of the digestive enzyme maltase-glucoamylase is to catalyze the hydrolysis of starch. It has the ability to break down starch into glucose, and later into small glucose units.
This enzyme is an efficient source of fermentation, and is used for both brewing beer and sake.
Maltase deficiency
The clinical importance of maltase and isomaltase is due to their crucial activity in the digestion of food starches and free absorbable glucose. Diseases related to maltase are Pompe disease, Alzheimer's and intolerance to the enzyme; all due to its deficiency. Results on the lack of disaccharides related to maltase enzyme activity deficiency are reported by numerous clinical laboratories using modified Dahlqvist assays.
Pompe disease
In 1932, Dutch pathologist Johannes Cassinus Pompe performed an autopsy on a 7-month-old girl who had died of no known cause. When performing it, he was able to observe a massive accumulation of glycogen in her body tissues. He later discovered that the cause of glycogen agglutination was the deficiency of the enzyme acid α-glucosidase or acid maltase whose function is to hydrolyze glycogen producing glucose within the cell lysosome. It was in this way that the first case of what is known today as Pompe disease was identified.
This anomaly seriously affects the normal metabolism of glycogen, an essential polysaccharide since it is the source of energy for animals. After hydrolysis, maltose, isomaltose and glucose are obtained. This last molecule is the basic molecule for life, and its insufficiency leads to numerous problems. The inability to hydrolyze glycogen results in glycogen storage in lysosomes.
Maltase deficiency is a genetically determined abnormality caused by mutations in the GAA gene. Pompe disease is inherited in an autosomal recessive fashion, which means that the parents carry mutations in the GAA gene, but do not have the disease.
Pompe disease affects 1 in 40,000 live births worldwide. Therefore it is considered a rare disease.
For a long time, this disease was considered incurable, but the approval of enzyme replacement therapy (EST) has opened the search for new cures for the disease. Thanks to this development, Pompe disease has joined the few lysosomal storage disorders for which treatment is already a reality.
Maltase intolerance
Intolerance to maltase, which requires the simultaneous absence of 4 autonomous enzymes, is usually accompanied by intolerance of sucrose and isomaltose. Maltase intolerance causes a problem of intestinal malabsorption, since the organism is unable to to absorb certain nutrients without the necessary catalytic proteins.
According to a study carried out by the Gastroenterology Unit of the Faculty of Clinical Medicine of Santiago de Chile, intestinal malabsorption of carbohydrates is caused by a lack of enzymes responsible for metabolizing sugars, which can be either due to a lack of this type of enzymes or due to a decrease in their activity. Therefore, individuals who have maltase malfunction, as well as other vital enzymes such as amylase or lactase, will have a higher chance of developing carbohydrate malabsorption syndrome.
Alzheimer's
Reduced proportions of the AMY1A gene in the brain have been shown to increase the risk of Alzheimer's disease. Thus, alpha-amylase 1 is thought to be related to this condition. The alpha-amylase 1 enzyme is found primarily in saliva, where it breaks down polysaccharides in food, such as starch. The activity of the enzyme corresponds to the number of copies of the gene for salivary alpha-amylase (AMYA1), which varies greatly between individuals. Furthermore, this enzyme is found in other organs, such as the lungs, the heart, the ovaries or the intestine, and it has recently been discovered in the hippocampus.
Likewise, the presence of glycogen in the neuronal synapse has been demonstrated, therefore the alpha-amylase enzyme plays an important role in the degradation of synaptic glycogen, and consequently in the formation of memories. Although this idea is not yet confirmed, support for the hypothesis was found when a reduction or even complete loss of hippocampal synaptic α-amylase immunoreactivity was observed in patients with Alzheimer's dementia.
Contenido relacionado
Mustard
Restriction enzyme
Oxytocin