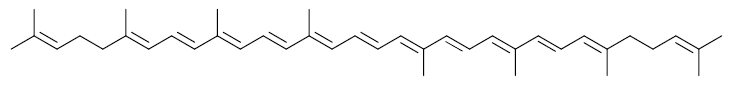
Lycopene
Lycopene from the Neo-Latin lycopersicum is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red-colored fruits and vegetables, such as carrots red bell peppers, watermelons, and papayas, although not strawberries or cherries. Although lycopene is chemically a carotene, it has no vitamin A activity. Foods that are not red in color may also contain lycopene, such as brown beans or parsley.
In plants, algae, and other photosynthetic organisms, lycopene is an important intermediate in the biosynthesis of many carotenoids, including beta carotene, which is responsible for yellow, orange, or red pigmentation, photosynthesis, and photosynthesis. -protection. Like all carotenoids, lycopene is a polyunsaturated hydrocarbon, that is, an unsubstituted alkene. Structurally, lycopene is a tetraterpene and assembled from eight isoprene units that are made up entirely of carbon and hydrogen. It is insoluble in water. Lycopene's eleven conjugated double bonds give it its deep red color and its antioxidant activity. Due to its strong color and non-toxicity, lycopene is a useful food color (registered E160d) and is approved for use in the US, Australia, and New Zealand (registered E160d). b>160d) and the EU.
Human consumption
Lycopene is not an essential nutrient for humans, but is commonly found in the diet primarily from tomato-based dishes. When absorbed from the intestine, lycopene is transported in the blood by various lipoproteins and it accumulates mainly in the blood, adipose tissue, skin, liver, and adrenal glands, but can be found in most tissues.
Preliminary research has shown that people who eat tomatoes may have a lower risk of cancer, possibly because lycopene affects the mechanisms of prostate cancer. However, this area of research and the relationship between lycopene and prostate cancer have been deemed insufficient evidence for approval of a "health claim" by the FDA.
Chemical composition of lycopene
Lycopene is one of the first carotenoids to appear in the synthesis of this type of compound, constituting the molecular basis for the synthesis of the remaining carotenoids. Lycopene is a carotenoid with a simple structure with an aliphatic chain made up of forty carbon atoms. Lycopene is a highly lipophilic carotenoid characterized by lacking cyclic rings and having a large number of conjugated double bonds. Its production by chemical synthesis is not yet fully established and, unlike other carotenoids such as β-carotene produced on a large scale by synthesis, lycopene is mainly obtained from natural sources, fungi and especially tomatoes. However, the extraction systems are expensive and lycopene has low stability, which has limited its use as a food coloring.
Sources of Lycopene
In our diet we obtain lycopene from very defined foods, mainly through the consumption of tomatoes and derivatives (sauces, fried tomatoes, crushed tomatoes, ketchup, pizzas, juices) and watermelon. In ripe tomatoes, the main carotenoid is lycopene, which contains approximately 83% of it, and in an equally important percentage, there is β-carotene, between 3-7%, and others such as γ-carotene, which like β-carotene, they have provitamin A, phytoene, phytofluene, etc. The lycopene content increases with the ripening of the tomatoes and can present great variations depending on the variety, crop conditions such as the type of soil and climate, type of storage, etc. The amount of lycopene in salad tomatoes is around 3000 µg/100g and in "pear type" is more than ten times that figure. In general, the lycopene content is lower in greenhouse-grown tomatoes, in any season, than in tomatoes grown outdoors during the summer, as well as the lycopene content is lower in fruits that are harvested green and ripe. in storage compared to the fruits ripened in the tomato plant.
It is currently possible to obtain, through genetic engineering, tomatoes that contain more than three times the amount of lycopene than other tomatoes.
The ease with which we incorporate lycopene into our body, that is, its bioavailability, is different depending on the way we consume it, so for example when it is taken with oil its absorption is facilitated. Research confirms that the intestinal absorption of lycopene is much better (up to 2.5 times more) if it is consumed when heated like sauces than as natural fruit or juice, because lycopene is better absorbed through fats and oils due to their lipid solubility and because, with high temperatures, the cell walls of the fruit break, which are what hinder the absorption of lycopene. Lycopene is present in the human organism both in blood in an amount of 30 µg/dl and in tissues, with a variable distribution. Lycopene is the predominant carotenoid in the composition of human tissues, concentrating especially in the prostate, which could explain its strong preventive action in the appearance of prostate cancer.
Mechanism of action of lycopene
Lycopene has antioxidant properties, and works by protecting human cells from oxidative stress, produced by the action of free radicals, which are one of the main causes of cardiovascular diseases, cancer and aging. In addition, It works by modulating the molecules responsible for regulating the cell cycle and producing a regression of certain cancerous and prostate lesions. The biological and physicochemical bases of these properties are not exactly known, but they seem directly related to the high antioxidant power of lycopene, much more than other antioxidants such as vitamin E or β-carotene. A large number of carcinogenic and degenerative processes are associated with oxidative damage to the genome and the genetic mechanisms that control cell proliferation and differentiation. Lycopene would act as a powerful neutralizer of free radicals (oxide and peroxide) attenuating oxidative damage to tissues.
Benefits of lycopene
There are more and more epidemiological studies that suggest that the consumption of lycopene has a beneficial effect on human health, significantly reducing the incidence of cancer pathologies, especially lung, prostate and digestive tract, cardiovascular and aging diseases. There is also scientific evidence that it prevents macular degeneration syndrome, the main cause of blindness in people over 65 years of age.
A study carried out by researchers at Harvard University revealed that the consumption of lycopene reduced the chances of developing prostate cancer by 45% in a population of 48,000 subjects who had at least 10 weekly servings in their diet. tomato or its by-products. The investigation lasted six years. Other research found that lycopene also reduces cholesterol levels in the form of low-density lipoprotein (LDL), which causes atherosclerosis, so tomato intake reduces the incidence of cardiovascular disease.
The first studies focused on the benefits they provided in the prevention of certain cancers, they showed that those people who consumed it frequently were less exposed to cancers that affected the digestive and reproductive systems, such as colon and prostate cancer..
Other later ones came to demonstrate the anti-aging properties of lycopene. An example is the one carried out with a group of 90 nuns, in southern Italy, aged between 77 and 98 years. Those with higher levels of lycopene in the blood had greater agility when performing all kinds of activities.
It is estimated that in Spain, from fresh fruits and vegetables, the amount of lycopene consumed is approximately 1.3 mg/person/day.
The fact that there is a lot of evidence that shows that the lycopene contained in our diet is beneficial for our health, does not mean that if we ingest it in isolation in the form of pills or capsules it will improve our health or we can avoid certain diseases. Many studies would still have to be carried out before recommendations can be made to consume it on its own as a dietary supplement. But what can be recommended is to increase your intake from fruits and vegetables.
Usefulness of lycopene
No toxicity problems have been described with an increase in the dietary intake of lycopene, except in carotenoderma. One must be somewhat skeptical of the "promising" perspectives derived from the different types of epidemiological studies, since there are several aspects that need more information, such as:
- A greater study in relation to its functions or activities in the human organism, since there are very few studies in people using lycopene in amounts higher than usual in the food, as well as for periods of prolonged time.
- Epidemiological studies cannot establish causal relationships; experimental studies are needed.
- It is necessary to remember the negative results that intervention with other carotenoids in certain population groups, for example β-carotene used in the prevention of lung cancer in smokers, with the result of increased incidence of this disease.
- The bioavailability of lycopene is quite variable according to its contribution form and its potential benefit could be the result of complex interactions between several carotenoids, vitamins and other dietary components.
- There is no scientific evidence of supplementation with lycopene in our diet, nor what is the correct dose, nor what population group to administer it, nor the duration of such supplementation.
Lycopene as a dye
Because it is so common, lycopene has been allowed to be used as a food coloring. Due to lycopene's insolubility in water and its tight binding to plant fiber, its availability has increased with the use of processed foods. For example, cooking tomatoes for casseroles or casseroles (similar to canned tomato sauces) and serving them in dishes rich in oils (such as pasta or pizza sauces) increases the assimilation of lycopene into the bloodstream.
Lycopene instantly stains any medium porous surface, including most plastics. While tomato stains can be easily cleaned from fabrics (while the stains are still wet), stained plastics defy all efforts to remove lycopene with hot water, soaps, or detergents (although bleach products destroy it). Plastics are especially susceptible to staining if they are heated, scratched, soaked in oil, or attacked by acids (such as those found in tomatoes).
Contenido relacionado
Potassium
Theodor Schwann
Primates