Liquid
The liquid is a state of aggregation of matter in the form of a highly incompressible fluid, which means that its volume is almost constant over a large range of pressure. It is the only state with a definite volume, but not fixed shape. Liquid is made up of tiny vibrating particles of matter, such as atoms and molecules, held together by intermolecular bonds.
Although water is the most common liquid on Earth, as well as the most abundant, this state of matter is actually the least common in the known universe, because liquids require a relatively large temperature/pressure range. narrow to exist. Most of the known matter in the universe is in gaseous form (with traces of detectable solid matter) as interstellar clouds or as plasma inside stars.
Like a gas, a liquid is able to flow and take the shape of a container. Unlike a gas, a liquid does not disperse to fill every space in a container, but it does maintain a constant density. The density of a liquid is usually close to that of a solid and much higher than that of a gas. Therefore, both liquid and solid are called condensed matter. A distinctive feature of the liquid state is surface tension, giving rise to wetting phenomena.
Description of liquids
The liquid state is an intermediate state of aggregation of matter between the solid state and the gas state. The molecules of liquids are not as close together as those of solids, but they are less far apart than those of gases. Molecules in the liquid state occupy random positions that vary with time. Intermolecular distances are constant within a narrow range. In some liquids, the molecules have a preferred orientation, causing the liquid to exhibit anisotropic properties (properties, such as refractive index, that vary with direction within the material).
Liquids have surface tension and capillarity, they generally expand when their temperature increases and lose volume when they cool down, although under compression their volume is very little variable, unlike what happens with other fluids such as gases. Objects immersed in any liquid are subject to a phenomenon known as buoyancy.
Liquid State
Its shape is spherical if no external force acts on it. For example, a drop of water in free fall takes the spherical shape.
As a fluid subject to the force of gravity, the shape of a liquid is defined by its container. In a liquid at rest subject to gravity, at any point within it there is a pressure of equal magnitude towards all sides, as established by Pascal's principle. If a liquid is at rest, the hydrostatic pressure at any point in it is given by:
- p=ρ ρ gz{displaystyle p=rho gz,}
Where ρ ρ {displaystyle rho } It's fluid density, g{displaystyle g} is gravity (9.8 m/s) and z{displaystyle z} is the distance of the point considered to the free surface of the liquid at rest. In a motion fluid the pressure is not necessarily isotropa, because hydrostatic pressure adds the hydrodynamic pressure that depends on the speed of the fluid at each point.
Status changes
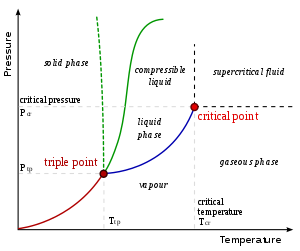
Under appropriate conditions of temperature and pressure, most substances can exist in a liquid state. When a liquid exceeds its boiling point it changes its state to a gas, and when it reaches its freezing point it changes to a solid. Although at atmospheric pressure, however, some solids sublime on heating; that is, they pass directly from the solid state to the gaseous state (see evaporation). The density of liquids is usually somewhat less than the density of the same substance in a solid state. Some substances, such as water, are denser in the liquid state.
Through fractional distillation, liquids can be separated from one another as each evaporates upon reaching its respective boiling point. The cohesion between the molecules of a liquid is not strong enough so that the surface molecules can evaporate.
Properties of Hydrostatics
Viscosity
Liquids are characterized because their internal forces do not depend on the total deformation, although they usually do depend on the deformation speed, this is what differentiates deformable solids from liquids. Real fluids are characterized by having a resistance to flow called viscosity (which is also present in viscoelastic solids). This means that in practice to maintain the speed in a liquid it is necessary to apply a force or pressure, and if this force stops the movement of the fluid finally stops after a finite time.
The viscosity of a liquid increases with increasing molar mass and decreases with increasing temperature. Viscosity is also related to the complexity of the molecules that make up the liquid: it is low in liquefied inert gases and high in heavy oils. It is a characteristic property of all fluids (liquids or gases).
Viscosity is a measure of the resistance to displacement of a fluid when there is a difference in pressure. When a liquid or a gas flows, it is assumed that there is a stationary layer of liquid or gas attached to the surface of the material through which the flow occurs. The second layer rubs against the one adhered superficially and this second with a third and so on. This friction between the successive layers is responsible for the opposition to the flow, that is, it is responsible for the viscosity.
Viscosity is measured in poises, one poise being the viscosity of a liquid in which to slide a layer of one square centimeter in area at a speed of 1 cm/s with respect to another stationary one located 1 cm away. the force of a dyne is needed.
Viscosity usually decreases in liquids with increasing temperature, although a few liquids exhibit an increase in viscosity when heated. For gases, the viscosity increases with increasing temperature.
The viscosity of a liquid is determined by means of a viscometer, among which the most widely used is the Ostwald viscometer. This is used to determine relative viscosity, that is, knowing the viscosity of a standard liquid, generally water, The viscosity of the problem liquid is obtained from the equation:
♫n1n2=d1× × t1d2× × t2♫{displaystyle ''{n_{1} over n_{2}}}={frac {d_{1}times t_{1}{d_{2}times t_{2}}}}}}}'
The viscous behavior of a liquid can be Newtonian or non-Newtonian. A Newtonian fluid exhibits a linear strain/stress curve, which means that its viscosity is independent of time, shear rate, or shear rate history. Examples of Newtonian liquids include water, glycerin, motor oil, honey, or mercury. A non-Newtonian liquid is one in which the viscosity is not independent of these factors and thickens (viscosity increases) or thins (viscosity decreases) under shear stresses. Examples of non-Newtonian liquids include ketchup, mayonnaise, hair gel, play dough, or starch solutions.
Fluency
Fluidity is a characteristic of liquids or gases that gives them the ability to pass through any orifice or hole, no matter how small, as long as it is at the same level as the container in which the liquid is found, unlike of the remaining state of aggregation known as solid.
Fluidity is due to the fact that a fluid can acquire an arbitrarily large deformation without the need to exert mechanical stress. The mechanical stress or pressure within the fluid depends essentially on the speed of the deformation, not on the deformation itself, unlike solids that have shape memory and experience greater stress the further away they are from the original shape. that is, in a solid, stress is primarily related to the degree of deformation.
Vapor pressure
Pressure of a vapor in equilibrium with its liquid form, the so-called vapor pressure, only depends on the temperature; its value at a given temperature is a characteristic property of all liquids.
So are the boiling point, freezing point, and heat of vaporization (essentially, the heat required to transform a given amount of liquid into vapor).
Under certain conditions, a liquid can be heated above its boiling point; liquids in that state are called superheated. It is also possible to cool a liquid below its freezing point and it is then called a supercooled liquid.
Other properties
Liquids do not have a fixed shape but do have volume. They have shape variability and very particular characteristics that are:
- Cohesion: force of attraction between equal molecules
- Accession: force of attraction between solid state molecules and atmospheric viscosity
- Surface tension: force manifested on the surface of a liquid, through which the outer layer of the liquid tends to contain the volume of the liquid within a minimum surface.
- Capilarity: ease of fluids to go up through tubes of tiny diameters (capills) where the cohesion force is overcome by the adhesion force.
Uses
Liquids have a variety of uses, including beverages, lubricants, solvents, and coolants. In hydraulic systems, fluid is used to transmit power.
In tribology, liquids are studied for their properties as lubricants. Lubricants such as oil are chosen for their viscosity and flow characteristics that are suitable over the entire operating temperature range of the component. The oils are often used in motors, gearboxes, metalworking, and hydraulic systems for their good lubricating properties.
Many liquids are used as solvents to dissolve other liquids or solids. Solutions are found in a wide variety of applications, including paints, sealants, and adhesives. Naphtha and acetone are frequently used in industry to clean oil, grease, and tar from parts and machinery. Body fluids are water-based solutions.
Surfactants are commonly found in soaps and detergents. Solvents such as alcohol are often used as antimicrobials. They are found in liquid dye cosmetics, inks, and lasers. They are used in the food industry, in processes such as the extraction of vegetable oil.
Liquids tend to have better thermal conductivity than gases, and the ability to flow makes a liquid well-suited for removing excess heat from mechanical components. The heat can be removed by channeling the liquid through a heat exchanger, such as a radiator, or the heat can be removed with the liquid during evaporation. Water or glycol coolants are used to prevent engines from overheating. Coolants used in nuclear reactors include water or liquid metals, such as sodium or bismuth salts. Liquid propellant Films are used to cool thrust chambers from rockets. In machining, water and oils are used to remove excess heat generated, which can quickly ruin both the workpiece and tooling. During perspiration, sweat removes heat from the human body by evaporation. In the heating, ventilating, and air conditioning industry, liquids such as water are used to transfer heat from one area to another.
Similarly, liquids are often used in cooking for their better heat transfer properties. In addition to better conductivity, because hotter fluids expand and rise while cooler areas contract and sink, liquids with low kinematic viscosity tend to transfer heat by convection at a fairly constant temperature, making that a liquid is suitable for scalding, boiling or frying. Even higher rates of heat transfer can be achieved by condensing a gas into a liquid. At the boiling point of the liquid, all the thermal energy is used to cause the phase change from liquid to gas, without a consequent increase in temperature, and is stored as chemical potential energy. When the gas condenses back into a liquid, this excess heat energy is released at a constant temperature. This phenomenon is used in processes such as steaming. Since liquids often have different boiling points, mixtures or solutions of liquids or gases can typically be separated by distillation, using heat, cold, vacuum, pressure, or other means. Distillation can be found in everything from the production of alcoholic beverages, to oil refineries, to the cryogenic distillation of gases like argon, oxygen, nitrogen, neon, or xenon by liquefaction (cooling them below their individual boiling points)..
Fluid is the main component of hydraulic systems, which take advantage of Pascal's law to provide fluid power. Devices such as pumps and water wheels have been used to transform the movement of liquids into mechanical work since ancient times. The oils are forced through hydraulic pumps, which transmit this force to the hydraulic cylinders. Hydraulics can be found in many applications, such as automobile brakes and transmissions, heavy equipment, and aircraft control systems. Various hydraulic presses are widely used in repair and manufacturing, for lifting, pressing, clamping, and forming.
Liquids are sometimes used in measuring devices. A thermometer often uses the thermal expansion of liquids, such as mercury, combined with their ability to flow to indicate temperature. A manometer uses the weight of the liquid to indicate the air pressure.
Microscopic description
The molecules that make up liquids are disordered and strongly interact, making liquids difficult to rigorously describe at the molecular level. This is in contrast to the other two common phases of matter, gases and solids. Although gases are disordered, they are dilute enough to ignore many-body interactions, and instead molecular interactions can be modeled in terms of well-defined binary collision events. By contrast, although solids are dense and strongly interacting, their regular structure at the molecular level (for example, a crystal lattice) allows for significant theoretical simplifications. For these reasons, the microscopic theory of liquids is less developed than that of gases and solids.
Static structure factor
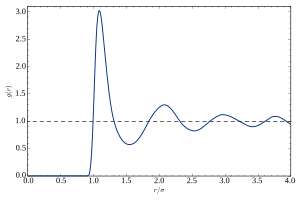
In a liquid, the atoms do not form a crystal lattice or show any other form of long-range order. This is evidenced by the absence of Bragg peaks in X-ray and neutron diffraction. Under normal conditions, the diffraction pattern has circular symmetry, expressing the isotropy of the liquid. In the radial direction, the diffraction intensity oscillates smoothly. This is usually described by the static structure factor S(q) with the wavenumber q = (4π/λ) sin θ given by the wavelength λ of the probe (photon or neutron) and the Bragg angle θ. The oscillations of S(q) express the near order of the liquid, that is, the correlations between an atom and some nearest neighbor shells.
A more intuitive description of these correlations is given by the radial distribution function g(r) which is basically the Fourier transform of S(q). It represents a spatial average of a temporal snapshot of pairwise correlations in the liquid.
Sound dispersion and structural relaxation
The previous expression for sound speed c=K/ρ ρ {displaystyle c={sqrt {K/rho }}}} contains the K-comprehension module. If K is independent of the frequency, then the liquid behaves like a linear medium, so the sound spreads without dissipation and without coupling. In fact, any liquid shows some dispersion: with a growing frequency, K crosses from the limit similar to the low frequency fluid K0{displaystyle K_{0}} up to the high frequency solid limit K∞ ∞ {displaystyle K_{infty}}. In normal fluids, most of this crossing takes place in frequencies between GHz and THz, sometimes called hypersonide.
At sub-GHz frequencies, a normal fluid cannot hold cutting waves: the zero frequency limit of the cutting module is G0=0{displaystyle G_{0}=0}. This is sometimes considered the defining property of a liquid. However, like the volume module K the cutting module G depends on the frequency, and in hypersonage frequencies shows a similar cross from the liquid limit G0{displaystyle G_{0}} to a solid limit, different from zero G∞ ∞ {displaystyle G_{infty }.
According to the Kramers-Kronig relation, the dispersion in the speed of sound (given by the real part of K or G) is accompanied by a maximum in the sound attenuation (dissipation, given by the imaginary part of K or G). According to linear response theory, the Fourier transform of K or G describes how the system returns to equilibrium after an external disturbance; for this reason, the scattering step in the GHz..THz region is also called structural relaxation. According to the fluctuation-dissipation theorem, relaxation towards equilibrium is closely related to fluctuations in equilibrium. The density fluctuations associated with sound waves can be observed experimentally using Brillouin scattering.
On supercooling of a liquid towards the glass transition, the crossover from a liquid-like response to a solid response moves from GHz to MHz, kHz, Hz,...; equivalently, the characteristic structural relaxation time increases from ns to μs, ms, s,... This is the microscopic explanation of the aforementioned viscoelastic behavior of glass-forming liquids.
Effects of association
The mechanisms of atomic/molecular diffusion (or particle displacement) in solids are closely related to the mechanisms of viscous flow and solidification in liquid materials. Descriptions of viscosity in terms of "head space" within the liquid were modified as necessary to account for liquids whose molecules are known to be "associated" in the liquid state at ordinary temperatures. When several molecules combine to form a partner molecule, they enclose within a semi-rigid system a certain amount of space that was previously available as free space for mobile molecules. Therefore, viscosity increases on cooling due to the tendency of most substances to associate on cooling.
Similar arguments could be used to describe the effects of pressure on viscosity, where it can be assumed that viscosity is primarily a function of volume for liquids with finite compressibility. Therefore, an increase in viscosity is expected with increasing pressure. Also, if the volume is expanded by heat but reduced again by pressure, the viscosity remains the same.
The local tendency to orient molecules in small groups gives the liquid (as mentioned above) a certain degree of association. This association results in an "internal pressure" considerable within a liquid, which is due almost entirely to those molecules that, due to their low temporal velocities (following the Maxwell distribution) have joined with other molecules. The internal pressure between several of these molecules could correspond to that of a group of molecules in solid form.
Contenido relacionado
Degree Fahrenheit
Gram
Functional group