Lipid
In biology and biochemistry, a lipid is a macro biomolecule that is soluble in nonpolar solvents. Nonpolar solvents are typically hydrocarbons used to dissolve other hydrocarbon lipid molecules that do not dissolve. readily (or do not dissolve) in water, including fatty acids, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), monoglycerides, diglycerides, triglycerides, and phospholipids.
The functions of lipids include energy storage, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries as well as in nanotechnology.
Scientists sometimes define lipids as hydrophobic and/or small amphiphilic molecules; The amphiphilic nature of some lipids allows them to form structures such as vesicles, unilamellar/multilamellar lysosomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits: ketoacyl and isoprene groups. Using this approach, lipids can be divided into eight categories: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, polyketides (derived from condensation of ketoacyl units); sterol lipids and prenol lipids (derived from condensation of isoprene subunits).
Although the term "lipid" is sometimes used synonymously with fats, fats are a subgroup of lipids called triglycerides. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, monoglycerides, and phospholipids), as well as other sterol-containing metabolites such as cholesterol. Although humans and other mammals use several biosynthetic pathways both To break down and synthesize lipids, some essential lipids cannot be manufactured in this way and have to be obtained from the diet.
The two main types of lipids in the blood are cholesterol and triglycerides.
Regarding their purpose in the human body, lipids are of crucial importance for energy storage and cell membrane development.
If lipid levels become too high, they can build up on artery walls to form plaque that can block blood flow.
History
Lipids can be considered as organic substances relatively insoluble in water, soluble in organic solvents (alcohol, ether etc.) actually or potentially related to fatty acids and used by living cells.
In 1815, Henri Braconnot classified lipids (graisses) into two categories, suifs (tallow or solid fats) and huiles (fluid oils). In 1823, Michel Eugène Chevreul developed a more detailed classification, including oils, fats, tallow, waxes, resins, balms, and volatile oils (or essential oils).
The first synthetic triglyceride was reported by Théophile-Jules Pelouze in 1844, when he produced tributyrin by treating butyric acid with glycerin in the presence of concentrated sulfuric acid. Several years later, Marcellin Berthelot, one of Pelouze's students, synthesized tristearin and tripalmitin by reacting the fatty acid analogue with glycerin in the presence of hydrogen chloride gas at high temperature.
In 1827, William Prout recognized fat ('fatty' food stuffs), along with protein ('albuminous') and carbohydrates ('saccharin'), as important nutrients for humans and animals.
For a century, chemists considered "fats" as unique simple lipids they made from acids and glycerol (glycerides), but new forms were described later. Theodore Gobley (1847) discovered phospholipids in chicken eggs and mammalian brains, he called them 'lecithins'. Thudichum discovered some phospholipids (cephalin), glycolipids (cerebroside) and sphingolipids (sphingomyelin) in the human brain.
The terms lipoid, lipid have been used with different meanings from author to author. In 1912, Rosenbloom and Gies proposed the substitution of "lipoid" by "lipin". In 1920, Bloor introduced a new classification for "lipoids": simple lipoids (fats and waxes), compound lipoids (phospholipoids and glycolipids), and derivatives of lipoids. (fatty acids, alcohols, sterols).
The word lipid, which roots etymologically from Greek λίπος, lipos 'fat', was introduced in 1923 by the French pharmacologist Gabriel Bertrand. Bertrand included in the concept not only the traditional fats (glycerides), but also the "lipoids", with a complex constitution. Even though the word lipide was unanimously approved by the international commission of the Société de Chimie Biologique during the plenary session on July 3, 1923. The word lipide was later anglicized as lipid due to its pronunciation (&# 39;lɪpɪd). In French, the suffix -ide, from Ancient Greek -ίδης (meaning 'above' or 'descendant of'), is always pronounced (ɪd).
In 1947, T. P. Hilditch divided lipids into "simple lipids", with fats and waxes (true waxes, sterols, alcohols).
Lipids have been classified into eight categories by the Lipid MAPS consortium as follows:
Fatty acids
Fatty acids, or fatty acid fragments when part of a lipid, are a diverse group of molecules synthesized by chain elongation of acetyl-CoA first with malonyl-CoA or methylmalonyl-CoA groups, in a process called synthesis. of fatty acid. They are made of a hydrocarbon chain that ends with a carboxylic acid group; This arrangement gives the molecule a polar, hydrophilic ending, and a nonpolar, hydrophobic ending that is insoluble in water. The structure of fatty acids is one of the most fundamental categories of biological lipids and is generally used as building blocks of more structurally complex lipids. The carbon chain, typically between four and twenty-four carbons in length, may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen, and sulfur. If a fatty acid contains a double bond, there is the possibility of either cis or trans geometric isomerism, which significantly affects the configuration of the molecule. The cis double bonds cause the fatty acid chain to bend, an effect that is accentuated when there are more double bonds in the chain. Three double bonds at carbon-18 of linolenic acid, the most abundant fatty acid chain in plant thylakoid membranes, make these membranes highly fluid despite low environmental temperatures, and also makes that linolenic acid presents sharp peaks in the high resolution 13-C NMR spectra of chloroplasts. This makes it play an important role in the structure and function of cell membranes. The most frequent form in which fatty acids occur is the cis configuration, although the form >trans exists in some partially hydrogenated natural fats and oils.
Examples of biologically important fatty acids include the eicosanoids, derived mainly from arachidonic acid and eicosapentaenoic acid, that include prostaglandins, leukotrienes, and thromboxanes. Docosahexaenoic acid which is also important in biological systems, particularly eye protection. Other important lipid classes in the fatty acid category are fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as waxy esters, coenzyme A thioester fatty acid derivatives, ACP fatty acid thioester derivatives, and fatty acid carnitines. Fatty amides include N-acyl ethanolamines, such as the neurotransmitter cannabinoid anandamide.
Glycerolipids
Glycerolipids are composed of mono-, di-, and trisubstituted glycerols, they are better known as glycerol triester fatty acids, called triglycerides. The word "triacylglyceride" is sometimes used as a synonym for "triglyceride". In these compounds, the three hydroxyl groups of glycerol are each esterified, typically by a different fatty acid. Because they function as energy stores, these lipids comprise the majority of fat stored in animal tissues. Hydrolysis of the ester bonds of triglycerides and the release of glycerol and fatty acids from adipose tissue are the initial steps in fat metabolism.
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar fragments attached to glycerol via a glycosidic bond. Examples of structures in this category are the digalactosyldiacylglycerols found in the membranes of plants and seminolipids of mammalian sperm cells.
Glycerophospholipids
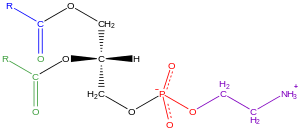
Glycerophospholipids, usually referred to as phospholipids (although sphingomyelin is also classified as phospholipids), are ubiquitous in nature and are key components of the cellular lipid bilayer, involved in metabolism and cellular communication. Neuronal tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in its composition have been associated with several neurological disorders. Glycerophospholipids can be subdivided into different classes, based on the polar nature of the group at the sn-3 position of glycerol. backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.
Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidyletaanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as the primary component of cell membranes and binding sites for intracellular and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acid, are precursors or derivatives of membrane second messengers. Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl bonds and linkages to long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.
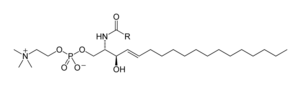
Sphingolipids
Sphingolipids are a complex family of compounds that share a common structural feature, a sphingoid base is synthesized de novo from the amino acid serine and a long fatty chain from acyl CoA, then converted to ceramides, phosphosphingolipids, glycosphingolipids, and other compounds. The main sphingoid base of mammals is generally referred to as sphingosine. Ceramides (Base N-acyl-sphingoid) are a major subclass of sphingoids derived from a fatty acid amide bond. Fatty acids are typically saturated or monounsaturated with chain lengths of 16 to 26 carbon atoms.
The important phosphosphingolipids of mammals are efingomyelins (ceramide, phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phosphoinositols and mannose-containing main groups. Glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
Sterols
Sterols, like cholesterol and its derivatives, are important components of membrane lipids, along with glycerophospholipids and sphingomyelins. Other examples of sterols are bile acids and their conjugates, which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter is also used as a biomarker for the growth of the algae. The predominant sterol in fungal cell membranes is ergosterol.
Sterols are steroids in which one of the hydrogen atoms is replaced with a hydroxyl group, at position 3 on the carbon chain. They have in common with steroids the same fused core four-ring structure. Steroids have different biological functions like hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family while the #C19 steroids comprise the androgens like testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids. The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring from the core structure.
Prenols
Prenol lipids are synthesized from precursors of five-carbon units of isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via mevalonic acid (MVA). Simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to the number of these terpene units. Large structures containing more than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors to vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid attached to the core quinonoid tail of non-isoprenoid origin. Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit remains bound to oxygen, while in animals polyprenols (dolichols) the isoprenoid terminal is reduced.
Saccharolipids
Saccharolipids describe compounds in which fatty acids are linked to a sugar skeleton, the conformation of this structure is compatible with membrane bilayers. In saccharolipids, a monosaccharide substitute for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipid is acylated glucosamine, a precursor to Lipid A, a component of lipopolysaccharide in Gram-negative bacteria. The typical composition of lipid A is glucosamine disaccharide molecules, which are derivatized from at most seven fatty-acyl chains. The minimum lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexaacylated disaccharide of glucosamine which is glycosylated with two 3-deoxy-D-mannno-octulosonic acid fragments (Kdo).
Polyketides
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classical enzymes, as well as iterative and multimodular enzymes that share mechanical characteristics with fatty acid synthases. They comprise many secondary metabolites and natural products of animal, plant, bacterial, fungal, and marine origin, and have great structural diversity. Many polyketides are cyclic molecules whose backbone chains are often further modified by glycosylation, methylation, hydroxylation, oxidation, or other processes. Many commonly used antimicrobial, antiparasitic, and anticancer agents are polyketides or derivatives of polyketides, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones.
Biological functions
Membranes
Eukaryotic cells present compartmentalized membrane-bound organelles that carry out different biological functions. The glycerophospholipids are the main structural component of biological membranes, as the cell plasma membrane and the intracellular membranes of organelles; In animal cells, the plasma membrane physically separates the intracellular components from the extracellular environment. [citation needed] Glycerophospholipid is an amphiphilic molecule (containing both hydrophobic and hydrophilic regions) that contains a glycerol core bound to two fatty acid-derived "tails& #3. 4; by ester connections and to a "group" head by a phosphate ester connection. [citation needed] While glycerophospholipids are the important component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes. In plants and algae, the galactosyldiacylglycerols, and sulfoquinovosyldiacylglycerol, both of which lack a phosphate group, are important components of membranes of chloroplasts and related organelles, and are the most abundant lipids in photosynthetic tissues, including those of higher plants, algae, and certain bacteria.
Thylakoid membranes have the largest lipid component of a non-bilayer monogalactosyl diglyceride (MGDG) conformation, and few phospholipids; Despite this unique lipid composition, thylankoid chloroplast membranes have been shown to contain a dynamic lipid bilayer matrix as revealed by magnetic resonance imaging and electron microscope studies.
A biological membrane is a lamellar phase form of a lipid bilayer. The formation of lipid bilayers is an energetically preferred process when the glycerophospholipids described above are in an aqueous environment. This is known as the hydrophobic effect. In an aqueous system, the polar lipid heads align toward the polar, aqueous environment, while the hydrophobic tails minimize their contact with water and tend to clump together, forming a vesicle; Depending on the lipid concentration, this biophysical interaction can result in the formation of micelles, liposomes, or lipid bilayers. Other aggregations are also observed and are part of the amphiphile (lipid) behavioral polymorphism. Phase behavior is an area of study within biophysics and is the subject of current [when?] academic research. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect. When a lipophilic or amphiphilic substance is dissolved in a polar environment, the polar molecules (i.e., water in aqueous solutions) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile.. So, in an aqueous environment, water molecules form a "clathrate" ordered around the dissolved lipophilic molecule.
The formation of lipids in protocell membranes represents a key step in models of abiogenesis, the origin of life.
Energy storage
Triglycerides, stored in adipose tissue, are an important form of energy storage in both animals and plants. They are an important source of energy because carbohydrates are fully reduced structures. Compared to glycogen which would contribute only half as much energy by its pure mass, the triglyceride carbons are all hydrogen bonded, unlike in carbohydrates. The adipocyte, or fat cell, for the continuous synthesis and breakdown of triglycerides in animals, with decomposition controlled mainly by the activation of enzymes sensitive to the hormone lipase. Complete oxidation of fatty acids provides high caloric content, approximately 38 kJ/g (9 kcal/g), compared to 17 kJ/g (4 kcal/g) for the breakdown of carbohydrates and proteins. Migratory birds that have to fly long distances without food use stored energy from triglycerides as power for their flights.
Signage
Evidence has emerged showing that lipid signaling is a vital part of cell signaling. Lipid signaling can occur through the activation of coupled proteins or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers. Among them is sphingosine-1-phosphate, a ceramide-derived sphingolipid that is a potent messenger molecule involved in the regulation of the calcium mobilization, cell growth, and apoptosis; diacylglycerol (DAG) and phosphatidylinositol phosphates (PIPs), which are involved in calcium-mediated activation of protein kinase C; prostaglandins, which are a type of eicosanoid derived from fatty acids that is involved in inflammation and immunity; steroid hormones such as estrogen, testosterone, and cortisol, which modulate a number of functions such as reproduction, metabolism and blood pressure; and oxysterols such as 25-hydroxycholesterol which are liver X receptor agonists. Phosphatidylserine lipids are known to be involved in signaling for phagocytosis of apoptotic cells or cell pieces. They achieve this by being exposed to the extracellular face of the cell membrane after the inactivation of flippases that place them exclusively on the cytosolic face and the activation of scramblases, which scramble the orientation of the phospholipids. After this occurs, other cells recognize the phosphatidylserines and engulf the cells or cell fragments that expose them.
Other functions
"Fat-soluble" (A, D, E, and K)—which are isoprene-based lipids—are essential nutrients that are stored in the liver and fatty tissues, with various functions. Acylcarnitines are involved in the transport and metabolism of fatty acids into and out of mitochondria, where they undergo beta-oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides to through the membranes. Polprenol phosphate sugars and polyprenol diphosphate sugars function in extracytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for example, peptidoglycan polymerization in bacteria), and in N-glycosylation of eukaryotic proteins. Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane. They are thought to activate enzymes involved in oxidative phosphorylation. The lipids also form the basis of steroid hormones.
Metabolism
The main lipids in the diet of humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The process of lipid metabolism synthesizes and degrades lipid stores and produces the structural and functional lipids characteristic of individual tissues.
Biosynthesis
In animals, when there is an excess supply of carbohydrates in the diet, the excess carbohydrates are converted to triglycerides. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of the fatty acids in the production of triglycerides, a process called lipogenesis. The fatty acids are manufactured by fatty acid synthases that polymerize and then reduce the acetyl units. -CoA. The acyl chains of fatty acids are extended by a cycle of reactions that add the acetyl group, reduce it to an alcohol, dehydrate it to an alkene group, and then reduce it back to an alkane group. Fatty acid biosynthesis enzymes are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein, while in the plastids of the fatty acids. plants and in bacteria separate enzymes perform each step of the pathway. Fatty acids can be further converted to triglycerides which are packaged into lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly unsaturated fatty acid linoleic acid as well as the triple unsaturated alpha-linolenic acid cannot be synthesized in mammalian tissues, so they are essential fatty acids and must be obtained from the diet.
Degradation
Beta oxidation is the metabolic process by which fatty acids are broken down in mitochondria or peroxisomes to generate acetyl-CoA. For the most part, fatty acids are oxidized by a mechanism that is similar, but not identical, to a reversal of the fatty acid synthesis process. That is, two-carbon fragments are sequentially removed from the carboxyl terminus of the acid after the dehydrogenation, hydration, and oxidation steps to form a beta-keto acid, which is cleaved by thiolysis. Acetyl-CoA is ultimately converted to ATP, CO2, and H2O via the citric acid cycle and electron transport chain. Thus, the citric acid cycle can begin in acetyl-CoA when fat is broken down for energy if little or no glucose is available. The energy yield for complete oxidation of the fatty acid palmitate is 106 ATP. Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation.
Nutrition and health
Most of the fat found in food is in the form of triglycerides, cholesterol, and phospholipids. Some dietary fats are necessary to facilitate the absorption of fat-soluble vitamins (A, D, E, and K) and carotenoids. Humans and other mammals have a dietary need for certain essential fatty acids, such as linoleic acid. (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) because they cannot be synthesized from simple dietary precursors. Both fatty acids are 18-carbon polyunsaturated fatty acids that differ in number and position of double bonds. Most vegetable oils are rich in linoleic acid ([safflower oil|safflower]], sunflower and corn). Alpha-linolenic acid is found in the green leaves of plants and in some seeds, nuts, and legumes (particularly flax, rapeseed, walnuts, and soybeans). Fish oils are especially rich in omega-3 fatty acids. long-chain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Many studies have shown positive health benefits associated with omega-3 fatty acid consumption on child development, cancer, cardiovascular disease, and various mental illnesses. (such as depression, attention deficit hyperactivity disorder, and dementia).
In contrast, it is now well established that the consumption of trans fats, such as those found in partially hydrogenated vegetable oil, is a risk factor for cardiovascular disease. Fats that are good for you can be converted to trans fats by improper cooking methods that result in overcooking of lipids.
A few studies have suggested that total dietary fat intake is associated with an increased risk of obesity and diabetes, however, a number of very large studies, including the Women's Health Initiative Dietary Modification Trial, an eight-year study of 49,000 women, the Nurses' The Health Study and the Health Professionals Follow-up Study revealed no such links. Neither of these studies suggested any connection between the percentage of calories from fat and the risk of cancer, heart disease, or weight gain. The Nutrition Source, a website maintained by the department of nutrition at Harvard University's T. H. Chan School of Public Health, summarizes the current evidence on the effect of dietary fat: "Detailed research — many of them conducted at Harvard—show that the total amount of fat in the diet is not really related to weight or disease".
Contenido relacionado
Biodiversity
Corvus
Galium aparine