Lactose
Lactose is a disaccharide formed by the union of a glucose molecule and a galactose molecule. It is also known as milk sugar, since it appears in the milk of the females of most mammals in a variable proportion between 4 and 6%.
Structure and chemical properties
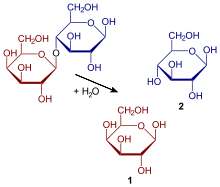
Specifically, its structure is β-D-galactopyranosyl-(1→4)-D-glucopyranose; the link involves carbon 1 of galactose (in beta configuration) and carbon 4 of glucose (both glucose anomers, α or β, can form lactose). When the bond between the two monosaccharides is formed, a water molecule is released. In addition, this compound has free hemiacetal hydroxyl (at carbon 1 of glucose), which is why it gives the Benedict reaction, that is, it is a reducing sugar.
It crystallizes with a molecule of water of hydration, so its formula is: C12H22O11 H2O, for this reason it can also be called lactose monohydrate. The molar mass of lactose monohydrate is 360.32 g/mol. The molar mass of anhydrous lactose is 342.30 g/mol.
Metabolism
Its synthesis is produced by the action of a galactosyl-transferase present in the mammary gland, whose specificity is modified by the presence of α-lactalbumin, which is specifically synthesized in response to hormonal signals that trigger lactation. Mammals that do not synthesize α-lactalbumin, such as seals, secrete milk that does not contain lactose.
Lactose metabolism has been extensively studied in lactic acid bacteria, given the economic relevance of products such as cheese and yogurt that result from the fermentation of lactose present in milk. Lactose can be transported by the sugar transport phosphotransferase system and metabolized by the tagatose-6-phosphate pathway or alternatively by a permease and metabolized by the Leloir pathway.
Absorption and intolerance
For the correct absorption of lactose, the presence in the intestine of the lactase enzyme, a β-galactosidase, is necessary. Mammals stop producing it after the first stage of life, with the exception of part of the human population (and some breeds of cats). These people are lactose tolerant, since milk consumption represented an evolutionary advantage. Lactose helps the absorption of calcium, allowing proper bone mineralization, and has prebiotic effects that benefit the intestinal flora.
When the body is not able to properly assimilate lactose, and depending on the amount consumed, various symptoms of lactose intolerance may appear, such as abdominal pain, bloating, rumbling, diarrhea, and even constipation and vomiting. However, the consumption of dairy products by people with lactose intolerance does not cause damage to the gastrointestinal tract, but is limited to these transient symptoms. A large proportion of people who believe they have lactose intolerance lactose do not actually have lactose malabsorption, but their symptoms are due to the presence of undiagnosed diseases (such as celiac disease, inflammatory bowel disease, or bacterial overgrowth) or a milk allergy, which is especially difficult to diagnose when it is not mediated by IgE. Healthy people (without diseases of the small intestine) with primary or permanent lactase deficiency can c Consume at least 12 g of lactose at each meal (the amount contained in a cup of milk) without experiencing any or only mild symptoms, and this tolerance is improved if milk is consumed with meals, choosing low-lactose milk, substituting milk for yogurt or aged cheeses, or by taking lactase supplements. Likewise, regular consumption of dairy foods by people with primary lactase deficiency may allow favorable adaptation of colonic bacteria, which may help to the breakdown of lactose, allowing a progressive and sustained tolerance to lactose.
Uses
Lactose's mild flavor and easy handling properties have led to the use of lactose as a carrier and stabilizer for flavors and pharmaceuticals. Lactose is not added directly to many foods, because its solubility is lower than that of other sugars commonly used in food. Infant formulas are a notable exception, where the addition of lactose is necessary to conform to the composition of human milk.
Lactose is not fermented by most yeasts during brewing, which can be exploited. For example, lactose can be used to sweeten stout; the resulting beer is often called milk stout or cream stout.
Yeasts belonging to the genus Kluyveromyces have a unique industrial application, since they are capable of fermenting lactose for the production of ethanol. Leftover lactose from whey is a potential alternative energy source.
Another important use of lactose is the pharmaceutical industry. Lactose is added to pharmaceutical tablets and capsules as an ingredient due to its physical and functional properties. For similar reasons it can be used to dilute illicit drugs such as cocaine or heroin.
History
The first isolation of lactose, carried out by the Italian physicist Fabrizio Bartoletti, was published in 1633. In 1700, the Venetian pharmacist Lodovico Testi published a pamphlet with recommendations on the use of milk sugar to alleviate, among other ailments, the symptoms of arthritis. In 1715, Testi's procedure for preparing milk sugar was published by Antonio Vallinesri. In 1780, Carl Wilhelm Scheele identified lactose as a carbohydrate.
In 1812, Heinrich Vogel observed that one of the products of lactose hydrolysis was glucose. In 1856, Louis Pasteur crystallized the other component of lactose, galactose. By 1894, Emil Fischer had established the configurations of the component monosaccharides.
Contenido relacionado
Campylobacter
Carex
Azo derivative