Joseph john thomson
Joseph John "J.J." Thomson, (English pronunciation: /ˈd͡ʒəʊzɪf d͡ʒɒn ˈtɒmsən/ ; Manchester, England, December 18, 1856-Cambridge, England, August 30, 1940) was a British scientist, discoverer of the electron, isotopes and inventor of the mass spectrometer. In 1906 he was awarded the Nobel Prize in Physics.
Biography
He was born on December 18, 1856 in Cheetham Hill, a district of Manchester in England, and was of Scottish ancestry. In 1870 he studied engineering at Owens College, now part of the University of Manchester, and transferred to Trinity College, Cambridge in 1876. In 1880, he obtained his Bachelor of Mathematics (Second Wrangler and second Smith Prize) and Master of Arts (winning the Adams Prize) in 1883. In 1884 he became Professor of Physics at Cavendish. One of his students was Ernest Rutherford, who would later be his successor in the position. Thomson was elected a Fellow of the Royal Society on June 12, 1884, and subsequently served as its President from 1915 to 1920. In 1897 he discovered the electron and proposed a model in which the electrons had negative charges and were inside the atom, which had a positive charge. Every April 30 marks the anniversary of the discovery of the first subatomic particle: the electron, an achievement that encyclopedias attribute to the Englishman Joseph John Thomson in 1897.
In 1890 he married Rose Elizabeth Paget, daughter of sir Edward George Paget, physician, then Regius Professor of Physic at Cambridge. With her, he fathered a son, George Paget Thomson, and a daughter, Joan Paget Thomson. His son became a noted physicist, who in turn was awarded the 1937 Nobel Prize in Physics for demonstrating the wave-like properties of electrons.
J. J. Thomson was awarded the Nobel Prize in Physics in 1906, "in recognition of the great merits of his theoretical and experimental investigations in the conduction of electricity generated by gases." He was knighted in 1908 and made an Order of Merit in 1912. In 1914 he gave the Romanes Lecture at Oxford on atomic theory. In 1918 he was appointed rector of Trinity College, Cambridge, where he met Niels Bohr, where he remained until his death. He died on August 30, 1940 and was entombed in Westminster Abbey, near sir Isaac Newton.
Works on cathode rays
Thomson performed a series of experiments on cathode ray tubes, which led to his discovery of electrons. Thomson used the Crookes tube in three different experiments.
Third experiment
In his third experiment (1897), Thomson determined the relationship between the charge and mass of cathode rays, by measuring how much they are deflected by a magnetic field and the amount of energy they carry. He found that the charge/mass ratio was more than a thousand times that of the hydrogen ion, suggesting that the particles are either very light or highly charged.
Thomson's conclusions were bold: cathode rays were made of particles he called 'corpuscles,' and these corpuscles came from inside the atoms of the electrodes, which means that the atoms are, of made, divisible. Thomson imagined that the atom is made up of these corpuscles in a sea full of positive charge; This model of the atom, attributed to Thomson, was called the raisin pudding model.
In 1906 he was awarded the Nobel Prize in Physics for his work on the conduction of electricity through gases.
The impossibility of explaining that the atom is made up of a compact nucleus and an outer part called the crust implies that other scientists such as Ernest Rutherford or Niels Bohr continued with their research and established other theories in which atoms had different parts
Discovery of isotopes
Thomson also invented positive rays and in 1911, discovered a way to use them to separate atoms of different masses. The objective was achieved by diverting the positive rays by means of electric and magnetic fields (mass spectrometry). He thus discovered that neon has two isotopes (neon-20 and neon-22).
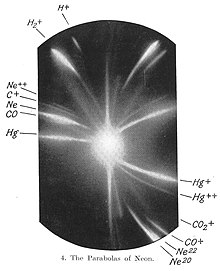
In the lower right corner of this photographic plate are markings for the two isotopes of neon, neon-20 and neon-22. In 1913, as part of his exploration into the composition of channel lightning, Thomson channeled a current of ionized neon through a magnetic field and an electric field and measured its deflection by placing a photographic plate in the path of the lightning. Thomson observed two patches of light on the photographic plate (see image to the right), which is two deflecting parabolas. Thomson concluded that neon gas is made up of two types of atoms of different atomic masses (neon-20 and neon-22).
Other jobs
Thomson in 1906 showed that hydrogen has a single electron. It allows confirming or rejecting various previous theories about the number of electrons, just like carbon.
Thomson proposed the second atomic model (the first was proposed by Dalton in 1794), which could be characterized as a positively charged sphere in which electrons are embedded.
He also analyzed the propagation of guided waves.
Awards
Apart from the Nobel Prize in Physics (1906), he was awarded the following prizes:
- Royal Medal (1894)
- Medalla Hughes (1902)
- Copley Medal (1914)
Legacy
In 1991, the thomson (Th) was proposed by chemists as a unit of mass-charge measurement in mass spectroscopy. This unit is defined as:
- 1Th=1ue=1Dae=1.036426× × 10− − 8kgC− − 1{displaystyle 1~mathrm {Th} =1~{frac {mathrm {u}{e}{e}}}{1~{mathrm {Da}}}}{e}}}}}=1.036426times 10^{-8}mathrm {kg,C^{1}}}}
However, it has become an obsolete unit, and has not been incorporated into the International System.
Eponymy
- The lunar crater Thomson carries this name in his memory.
Contenido relacionado
Physical
Physical constant
Wormhole