James Prescott Joule
James Prescott Joule (Salford, United Kingdom, December 24, 1818 – October 11, 1889) was an English physicist, one of the most notable physicists of his time, best known for his research in thermodynamics. He discovered his relation to mechanical work, which led him to the theory of energy. The international unit of energy, heat, and work, the joule, was named in his honor. He worked with Lord Kelvin to develop the absolute temperature scale, made observations on thermodynamic theory (Joule-Thomson effect), and found a relationship between electric current through a resistor and heat dissipated, now called Joule's law. After numerous experiments, he obtained the numerical value of the mechanical equivalent of heat. He helped explain the kinetic theory of gases. He was "possibly the last autodidact to make a significant contribution to the progress of science."
Biography
James Prescott Joule was the son of Benjamin Joule (1784-1858), a brewery owner. With a shy and humble character, he received private classes in his own home in physics and mathematics, being his teacher the British chemist John Dalton; He combined these classes with his professional activity, working with his father at the distillery, which he came to direct. Dalton encouraged him towards scientific inquiry and he conducted his first experiments in a laboratory near the brewery, while training at the University of Manchester. He only received two years of education in arithmetic and geometry before Dalton had to withdraw due to cerebrovascular accident. Even so, Dalton influenced Joule, as well as his associates, the chemist William Henry and the Manchester engineers Peter Ewart and Eaton Hodgkinson. Later, Joule was educated by John Davies. Joule was fascinated by electricity. He and his brother experimented with giving electric shocks to each other and also to the family servants.
Joule became manager of the brewery and took an active role until the business was sold in 1854. Science was a hobby, but he soon began to investigate the possibility of replacing the brewery's steam engine with an electric motor, then newly invented. Joule had a room in his father's house that he used as a laboratory; it was there that he began to do his first electrical and magnetic experiments.In 1838 he contributed his first scholarly papers on electricity to the Annals of Electricity , the scientific journal founded and edited by Davies' partner William Sturgeon. He discovered Joule's law in 1840 and tried to impress the Royal Society but discovered, not for the last time, that he was seen as a mere provincial novice. When Sturgeon moved to Manchester in 1840, he and Joule became the center of a circle of the city's intellectuals. Both shared the belief that science and theology had to be integrated. Joule taught at the Royal Victoria Gallery of Practical Science in Sturgeon.
He found that burning a pound of coal in a steam engine produces five times the work of burning a pound of zinc in a Grove Cell, an early electric battery. Joule's standard unit of work was the ability to lift one pound to a height of one foot, the foot-pound.
Joule was influenced by the thought of Franz Aepinus and tried to explain the phenomena of electricity and magnetism in terms of atoms surrounded by a "calorific ether in a state of vibration".
Even so, Joule's interest shifted from something purely financial about how much work could be extracted from a single source, to speculating about the transformation of energy. In 1843 he published the results of his experiments, which showed that the heating effect that he had quantified in 1841 was due to the generation of heat in the electrical conductor and not its transmission from another part of the equipment. This was a direct challenge of the caloric theory, which held that heat could neither be created nor destroyed. The caloric theory had dominated thought in the science of heat since Antoine Lavoisier had introduced it in 1783. Lavoisier's prestige and the practical success of Sadi Carnot's steam engine caloric theory since 1824 made the young Joule, who was working outside of the academic and engineering realms, had a difficult road to travel. Supporters of the caloric theory indicated the symmetry of the Peltier-Seebeck effect to affirm that heat and current are convertible, at least approximately, by a reversible process.
In June 1844, his father moved from Pendlebury to Whalley Range and had a laboratory built for his son near the house. Joule was closely associated with the Manchester Literary and Philosophical Society. On January 25, 1842 he became a member of the society, in 1844 he was appointed librarian, in 1846 honorary secretary, in 1851 vice president and finally, in 1860 he became president of the society for the first time.
The mechanical equivalent of heat
In his 1845 work, Joule wrote:
... mechanical work performed when rotating a magnetoelectric machine converts to heat created by the passage of inductive currents through its coils; and, on the other hand, that the motile power of the magnetoelectric engine is obtained at the expense of heat due to the chemical reactions of the battery that makes it work.
In this passage, Joule adopts the language of vis viva (energy), possibly because Hodgkinson had read a review of Ewart's On the measure of moving force to the Literary and Philosophical Society in April 1844.
Subsequent experiments and measurements by Joule led him to calculate that the mechanical equivalent of heat was 838 foot-pounds to raise the temperature of one pound of water by one degree Fahrenheit. He announced his results at a meeting of the Department of chemistry from the British Association for the Advancement of Science in Cork in 1843, but was met with silence in response.
Joule was not discouraged and began looking for a purely mechanical demonstration of the conversion of work into heat. By forcing water through a perforated cylinder, he was able to measure the slight viscous heating of the fluid. He got a mechanical equivalent of 770 foot-pounds/UTB (4.14 J/cal). The fact that the values obtained both by electrical and purely mechanical means corresponded by at least an order of magnitude was, for Joule, convincing evidence of the reality of the transformation of work into heat.
Joule tried a third way. He measured the heat generated by the work done in compressing a gas. He obtained a mechanical equivalent of 823 foot-pounds (4.43 J/cal.) In many ways, this experiment became the easiest target for Joule's critics, but Joule dealt with anticipated objections through clever experiments. Even so, his work was rejected by the Royal Society and he had to content himself with publishing it in the Philosophical Magazine . In this work his rejection of the caloric reasoning of Carnot and Émile Clapeyron was evident, but his theological motivations were also evident:
I believe that this theory is opposed to the recognized principles of philosophy because it leads to the conclusion that the living can be destroyed by an incorrect configuration of the apparatus: therefore, Mr. Clapeyron infers that "as the temperature of the fire is between 1000 °C and 2000 °C above the boiler, there is a huge loss of living in the heat passage from the oven to the boiler». Convinced that the power to destroy things belongs only to the Creator, I affirm... that any theory that, when put into practice, requires annihilation of force, is necessarily wrong.
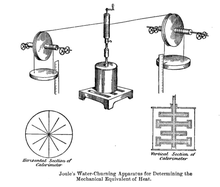
In 1845, Joule read his work On the mechanical equivalent of heat at the British Association meeting in Cambridge. In this work, he explained his best-known experiment, using a falling weight to make spinning a spool in an insulated water barrel, of which he measured the temperature rise. His new estimate of the mechanical equivalent was 819 foot-pounds/BTU (4.41 J/cal).
In 1850, Joule published a refined measurement of 772,692 foot-pounds/BTU (4,159 J/cal), closer to century estimates XX.
Reception and priority
Much of the initial resistance to Joule's work was due to its reliance on extremely precise measurements. He claimed to be able to measure temperatures to within 1/200 of a degree Fahrenheit. Accuracy of this magnitude was certainly rare in the experimental physics of his time, but critics of his might have dismissed his expertise in the art of brewing and his access to practical technologies.He also received support from the instrument maker scientists John Benjamin Dancer.
Even so, the German Hermann Helmholtz discovered Joule's work and a similar work published in 1842 by Julius Robert von Mayer. Although both had been despised since the publication of their respective papers, Helmholtz's 1847 definitive statement of the conservation of energy gave credence to both.
Also in 1847, one of the British Association's Joule exhibitions in Oxford was attended by George Gabriel Stokes, Michael Faraday, and the precocious and independent William Thomson, later Lord Kelvin, who had just won the Professor of Natural Philosophy at the University of Glasgow. Stokes was "inclined to be a Joulite" and Faraday "was very impressed," though he had his doubts. Thomson was intrigued but skeptical.
Thomson and Joule met unexpectedly later that year in Chamonix. Joule married Amelia Grimes on August 18, and the couple went on their honeymoon. Marital enthusiasm aside, Joule and Thomson decided to attempt an experiment a few days later to measure the difference in temperature between the top and bottom of the Sallanches Falls, though this later proved highly impractical..
Although Thomson felt that Joule's results demanded a theoretical explanation, he vigorously defended the school of Carnot and Clapeyron. In his 1848 treatise on absolute temperature, Thomson wrote that "the conversion of heat (or caloric) into a mechanical effect is probably impossible, and certainly has not been discovered" - but a footnote demonstrated his doubts about the absolute temperature. caloric theory, referring to Joule's "very remarkable discoveries." Surprisingly, Thomson did not send Joule a copy of the work, but when Joule finally read it, he wrote to Thomson on October 6, stating that his studies had shown the conversion of heat to work but that he was planning further experiments.. Thomson responded on the 27th, revealing that he was planning his own experiments and that he expected a reconciliation of the two theories from him. Although Thomson made no further experiments, over the next two years he became disenchanted with Carnot's theory and convinced of Joule's. In his 1852 work, Thomson wished to go no further than compromise and declared that "the whole theory of the motive power of heat is based on... two... propositions... due to Joule on the one hand, and Carnot and Clausius on the other." another".
As soon as Joule read the play, he wrote to Thomson with his comments and questions. Thus began a fruitful collaboration between the two men, despite the fact that it was essentially epistolary, with Joule carrying out experiments and Thomson analyzing the results and proposing new ones. The collaboration lasted from 1852 to 1856, and their discoveries included the Joule-Thomson effect, and the published results contributed much to the general acceptance of Joule's work and of the kinetic theory.
Kinetic theory
Kinetics is the science that studies motion. Joule was a student of Dalton's and not surprisingly, he developed a strong belief in atomic theory, even though many scientists of his day were still skeptical. He was also one of the few people receptive to John Herapath's little accepted work on the kinetic theory of gases. He was also deeply influenced by Peter Ewart's 1813 work On the measure of moving force.
Joule saw the relationship between his discoveries and the kinetic theory of heat. His lab notes reveal that he believed heat to be a rotational rather than a translational form of motion.
Joule couldn't resist finding antecedents for his beliefs in Francis Bacon, Isaac Newton, John Locke, Benjamin Thompson, and Humphry Davy. Although these beliefs are justified, Joule calculated a value for the mechanical equivalent of heat of 1,034 foot-pounds from Thompson's works. Some current scholars have criticized this, arguing that Thompson's experiments did not represent systematic quantitative measurements. In one of his personal notes, Joule argues that Mayer's measurement was no more precise than Thompson's, perhaps hoping that Mayer had not anticipated his work.
Death and acknowledgments
Joule was to have been the president of the British Association at the Bradford meeting in 1872 and again at the Manchester meeting in 1887, but on both occasions he was unable to attend for reasons of health. From 1872 his Health weakened, and from that time until his death on October 11, 1889, he lived quietly at his residence at 12 Wardle Road in Sale, where he studied as health permitted. He was buried in Brooklands Cemetery. The headstone bears the inscription "772.75", his culminating measure of the mechanical equivalent of heat (1878), and a quote from the Gospel of John, "As long as it is day, I must do the works of him who sent me: but now the night is drawing near, when no one can work" (John, 9:4).
Throughout his life, but especially during his last years, he received numerous awards from both England and abroad. Joule was elected a Fellow of the Royal Society on 6 June 1850. In 1852 the Council of the Royal Society awarded Joule the Royal Medal for research; in 1860 he was awarded the Copley Medal for the same experiments by Sir Edward Sabine; in the delivery speech Sabine said:
The award of two medals for the same research is an extremely rare procedure in our society, and rightly. On this occasion, the Council has wished to show as emphatically as possible its opinion of the special and original character and the great utility of Mr. Joule.Sir Edward Sabine (1860)
Several universities awarded him honorary doctorate degrees; the first was the D.C.L. by Trinity College Dublin (1857), then the D.C.L. by the University of Oxford (1860) and finally the D.C.L. from the University of Edinburgh in 1871. In 1878 he received a pension of £200 a year for services to science and in 1880 the Prince of Wales Edward VII presented him with the Albert Medal, awarded by the Royal Society of Arts. Joule has been an honorary member of the Institution of Engineers and Shipbuilders in Scotland since 1857.
Two oil portraits of James Prescott exist; the former is housed in a room of the Manchester Literary and Philosophical Society and was painted by George Patten in 1863, while the latter is owned by the Royal Society and is the work of George Reynolds in 1882. Reynolds also carved a bust in 1882.
In the city of Manchester, he has many recognitions, such as the library that bears his name and which, within the University of Manchester, is specialized in works of science, technology and engineering, or like the statue sculpted by Alfred Gilbert, located just before one of Dalton outside the town hall. At the heart of Westminster Abbey there is a monument to Joule, despite the fact that he is not buried there, contrary to what some biographies say.
Worthington Park, formerly Sale Park, is home to a bronze-plated clay bust depicting the figure of the scientist. The work is from 1905 by sculptor John Cassidy. The project was partially funded by donations, among others, from scientists around the world. The bust shows Joule holding a scientific work with a glimpse of a drawing of a galvanometer.
The lunar crater Joule is named after him, as well as the French submarine Joule, which sank in the Dardanelles in the spring of 1915.
Brewing activity
Together with his brother Benjamin, James Joule took over the Salford-based brewery that his grandfather William had founded three decades before his birth. While some authors claim that Joule had little involvement in running the brewery Others say the opposite, that Joule showed great interest in improving the technical, physical and chemical processes of the brewery. This latter point of view is supported by his correspondence with Lord Kelvin. The production of gas during the beer manufacturing process would have been the starting point for the studies that led to the discovery of the Joule-Thomson effect. The illness of his father and the departure of his brother forced James Joule to become involved with the technical and commercial aspects of the brewery. Joule ended up selling the brewery in 1855, a few years before his father's death, to focus exclusively on his scientific experiments.
Work
Joule studied aspects related to magnetism, especially those related to the magnetization of iron by the action of electric currents, which led him to the invention of the electric motor. He also discovered the phenomenon of magnetostriction, which appears in ferromagnetic materials, in which their length depends on their state of magnetization. But Joule's most fruitful area of research is related to the different forms of energy: with his experiments he verifies that when an electric current flows through a conductor, it experiences an increase in temperature; from there he deduced that if the source of electrical energy is an electrochemical battery, the energy would have to come from the transformation carried out by chemical reactions, which would convert it into electrical energy and from this it would be transformed into heat. If a new element is introduced into the circuit, the electric motor, mechanical energy is generated. This leads him to the enunciation of the principle of conservation of energy, and although there were other renowned physicists who contributed to the establishment of this principle such as William Thomson (Lord Kelvin) and Hermann von Helmholtz, it was Joule who gave it greater solidity.
In 1840 Joule published Production of heat by voltaic electricity, in which he established the law that bears his name and which states that the heat generated in a conductor by the passage of electric current is proportional to the product of the conductor resistance times the square of the current intensity. In 1843, after numerous experiments, he obtained the numerical value of the mechanical equivalent of heat, which he concluded was 4.15 J equal to one calorie (in today's units), which allowed conversion of mechanical and thermal units; This is a value very similar to that currently considered to be 4,187 J. In this way, the relationship between heat and work, already advanced by Thompson, was firmly established, which served as the cornerstone for the subsequent development of statistical thermodynamics. In these works Joule was based on the law of conservation of energy, discovered in 1842.
Despite the fact that in 1848 he had already published an article referring to the kinetic theory of gases, where for the first time the speed of gaseous molecules was estimated, he abandoned his line of research and preferred to become an assistant to William Thomson, and, as a result of this collaboration, the discovery of the Joule-Thomson effect was reached, according to which it is possible to cool an expanding gas if the necessary work is carried out to separate the gas molecules. This later made possible the liquefaction of gases and led to the law of internal energy of a perfect gas, according to which the internal energy of a perfect gas is independent of its volume and dependent on temperature.
Eponymy
- July (adaption to Spanish joule), energy unit of the International System, bears this name in your honor.
- The lunar crater Joule bears this name in his memory.
- The asteroid (12759) Joule also commemorates its name.
Contenido relacionado
Paradores of Tourism of Spain
William Bradford Shockley
Spanish Confederation of Autonomous Rights