Isotope
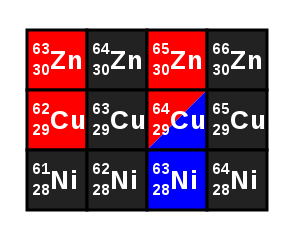
Isotopes are the atoms of the same element, whose nuclei have a different number of neutrons, and therefore, differ in mass number.
The word isotope (from the Greek: ἴσος isos 'same, same'; τόπος tópos 'place', &# 34;in the same place") is used to indicate that all types of atoms of the same chemical element (isotopes) are found in the same place in the periodic table. Atoms that are isotopes of each other are those that have the same atomic number (number of protons in the nucleus), but different mass number (sum of the number of neutrons and the number of protons in the nucleus). The different isotopes of an element therefore differ in the number of neutrons.
Most chemical elements have more than one isotope. Only 8 elements (for example beryllium or sodium) possess a single natural isotope. In contrast, tin is the element with the most stable isotopes, 10.
Other elements have natural but unstable isotopes, such as uranium, whose isotopes can change or decay into more stable isotopes, emitting radiation in the process, and are therefore said to be radioactive.
Unstable isotopes are useful for estimating the age of a wide variety of natural samples, such as rocks and organic matter. This is possible, as long as the average decay rate of a given isotope is known, relative to those that have already decayed. Thanks to this dating method, the age of the Earth can be estimated.
Types of isotopes
All isotopes of an element have the same atomic number but differ in what is now known as their mass number.
If the relationship between the number of protons and neutrons is not appropriate to obtain nuclear stability, the isotope is radioactive.
For example, in nature carbon occurs as a mixture of three isotopes with mass numbers 12, 13 and 14: 12C, 13C and 14C. Their abundances with respect to the global amount of carbon are respectively 98.89%, 1.11% and traces.
- Isotopes natural. Natural isotopes are those found in nature. For example, hydrogen has three natural isotopes, protio, deuterio and tritio. The trithium is widely used in nuclear-type jobs; it is the essential element of the hydrogen pump.
- Another element that is made up of very important isotopes is carbon, which is carbon-12, which is the baseline of the atomic weight of any element, carbon-13 which is the only carbon with magnetic properties and carbon-14 radioactive, very important since its semi-life is 5730 years and is used a lot in archaeology to determine the age of organic fossils. Uranium-235 is used in nuclear power plants and atomic bombs.
- Isotopes. The artificial isotopes are produced in nuclear laboratories by bombing subatomic particles or nuclear power plants. These isotopes usually have a short life, mainly due to the instability and radioactivity they present. One of these is the cesium, whose artificial isotopes are used in nuclear power generation plants. Another widely used is the iridium-192 that is used to check the hermetity of tube welds, especially in heavy crude and fuel transport tubes. Some uranium isotopes such as uranium-233 are also used in nuclear technology.
Isotopes are subdivided into stable isotopes (fewer than 300 exist) and non-stable or radioactive isotopes (there are about 1200). The concept of stability is not exact, since almost stable isotopes exist. Their stability is due to the fact that, although they are radioactive, they have an extremely long half-life compared to the age of the Earth.
| Isótopo | Nucles for million |
|---|---|
| Hydrogen-1 | 705 700 |
| Hydrogen -2 | 23 |
| Helio-4 | 275 200 |
| Helio-3 | 35 |
| Oxygen-16 | 5920 |
| Carbon-12 | 3032 |
| Carbon-13 | 37 |
| Neon-20 | 1548 |
| Neon-22 | 208 |
| Iron-56 | 1169 |
| Iron-54 | 72 |
| Iron-57 | 28 |
| Nitrogen-14 | 1105 |
| Silicio-28 | 653 |
| Silicio-29 | 34 |
| Silence-30 | 23 |
| Magnesium-24 | 513 |
| Magnesium-26 | 79 |
| Magnesium-25 | 69 |
| Azufre-32 | 39 |
| Argon-36 | 77 |
| Calcium-40 | 60 |
| Aluminium-27 | 58 |
| Nickel-58 | 49 |
| Sodium-23 | 33 |
Notation
Initially the names of the isotopes of each element that were being discovered received proper names different from the element to which they belonged. Thus when three isotopes of hydrogen were discovered, they received the names protium, deuterium, and tritium. The nucleus of protium consists of a proton, that of deuterium of a proton and a neutron, and that of tritium of a proton and two neutrons.
When isotopes of almost all the elements continued to be discovered, it was seen that hundreds or thousands of names would be necessary and the nomenclature system was changed. Currently, each isotope is represented with the symbol of the element to which it belongs, placing its atomic number (number of protons in the nucleus) as a subscript on the left, and its mass number (sum of the number of protons and neutrons) as a superscript on the left.). Thus the isotopes of hydrogen protium, deuterium, and tritium are denoted 11H, 12H, and 13H, respectively.
Since all the isotopes of the same element have the same atomic number, which is the order in the periodic table, and the same symbol, the atomic number is usually omitted. Thus for the isotopes of hydrogen we will write 1H, 2H and 3H. This is done because all isotopes of a particular element behave in the same way in any chemical reaction. For example, an atom of the rare isotope of oxygen, mass number 18, will combine with two hydrogen atoms to form water in exactly the same way as the abundant oxygen atom of mass number 16. However, when describing nuclear reactions it is useful to have the atomic number as a reference.
In the case of non-scientific texts, such as journalistic texts, this notation with subscripts and superscripts is cumbersome, so a notation consisting of the name of the element joined by a hyphen to the mass number of the isotope is also used. try. Thus the isotopes of hydrogen 11H, 12H and 1 >3H, can also be named hydrogen-1, hydrogen-2, and hydrogen-3 respectively.
These are the scientifically accepted nomenclature rules, corresponding to the Nomenclature of Inorganic Chemistry. 2005 Recommendations (IUPAC Red Book), as can be found in its section IR-3.3.
It must be remembered that the names of chemical elements are common names and as such must be written without an initial capital letter, unless another spelling rule dictates it.
Radioisotopes
Radioisotopes are radioactive isotopes in that they have an unstable atomic nucleus and give off energy and particles when they transform into a different, more stable isotope. The disintegration can be detected with a Geiger counter or with photographic film.
The main reason for instability is in the excess of protons or neutrons. The strong nuclear force, which binds protons and neutrons together, requires that the number of neutrons and protons be close to a certain ratio. When the number of neutrons is greater than that required by this relationship, the atom can exhibit negative beta decay. When the atom has an excess of protons (deficiency of neutrons) it usually presents positive beta decay.
This happens because the residual strong nuclear force depends on the ratio of neutrons to protons. If the relationship is very biased towards one of the extremes, the weak nuclear force responsible for beta decay can sporadically cause the loss of some nucleon. For high atomic numbers (Z > 80) alpha decay also becomes frequent (which is almost much more frequent when there is also an excess of protons).
Each radioisotope has a characteristic half-life or half-life. The energy can be released mainly in the form of alpha radiation (particles made up of helium nuclei), beta (particles made up of electrons or positrons) or gamma (energy in the form of electromagnetic radiation).
Several artificial and unstable radioactive isotopes have uses in radiation therapy techniques in medicine. For example, an isotope of technetium (99mTc, the "m" indicates that it is a metastable nuclear isomer) can be used to identify blocked blood vessels.
Several naturally occurring radioactive isotopes are used in radiometric dating to determine chronologies, for example, archaeological.
Applications of isotopes
The following are several of the applications of different isotopes in various areas, such as medicine:
- Cobalto-60. For cancer treatment because it emits a radiation with more energy than the one that emits the radio and is cheaper than this.
- Arsenico-73. It is used as a tracer to estimate the amount of arsenic absorbed by the body and arsenic-74 in the location of brain tumors.
- Bromo-82. Useful for hydrology studies such as water flow determination, water flow directions and residence times in surface and groundwater, determination of the dynamics of lakes and leaks in reservoirs.
- Gold-19. High application in the oil industry: drilling oil wells, secondary oil recovery studies, which are advanced in the determination of incremental production and petrochemical industry in general.
- Phosphorus-32. It is an isotope that emits beta rays and is used to diagnose and treat bone marrow-related diseases.
- Scandio-46. Applicable in sedimentology studies and soil analysis.
- Lantano-140. Used in the study of the behavior of boilers and furnaces used in the industrial sector.
- Mercury-197. Application in electrolytic cells.
- Nitrogen-15. It is often used in medical research and agriculture. It is also commonly used in nuclear magnetic resonance imaging (NMR).
- Yodo-131. He is one of the radionuclides involved in the atmospheric nuclear tests, which began in 1945. It increases the risk of cancer and possibly other thyroid diseases and those caused by thyroid hormone deficiencies.
- Radio-226. In treatments to cure skin cancer.
- Tritio, 3H. The trithium has medical applications as a tracker as it breaks down, as we have seen, emits low-energy electrons but not rays (which is a much more dangerous type of radiation). The hydrogen pump is actually tritio pump.
- Tecnecio-99. It can be used to identify blocked blood vessels.
- Oxygen-18 and Deuterior. These two isotopes are used very commonly to infer the temperature of the earth in the past.
- carbon-14 radioactive, very important since its semi-life is 5730 years and is used a lot in archaeology to determine the age of organic fossils.
- Uranium-235 is used in nuclear power plants and atomic bombs.
Using chemical properties
In isotopic labeling, unusual isotopes are used as markers for chemical reactions. The added isotopes react chemically the same as those present in the reaction, but can later be identified by mass spectrometry or infrared spectroscopy. If radioisotopes are used, they can also be detected thanks to the radiation they emit. Isotopic separation or isotopic enrichment processes represent a challenge.
Contenido relacionado
Aromatic compound
Natural Sciences
Vectorial space
