Isomerism
Isomerism is a property of those chemical compounds (especially carbon chains) that have the same molecular formula (chemical formula not developed) of equal relative proportions of the atoms that make up their molecule, but they have different chemical structures and therefore different properties and configuration. These compounds are called isomers. For example, ethyl alcohol or ethanol and dimethyl ether are isomers whose molecular formula is C2H6O.
In fact, one of the first things a chemist has to do when he wants to solve the complete structure of a new molecular species is to establish the chemical elements that are part of its composition and also the relative proportion in which the elements are found. themselves.
Then you must determine the molecular weight of the new chemical substance and from all this data calculate the molecular formula, that is, the number of atoms of each element that are present in the main molecule of the hydrocarbon.
At this point, research should be focused on determining the way in which the atoms are connected to each other in the molecule under study and how these atoms are arranged in space, at which point isomerism comes into play, since numerous possibilities of substances with the same molecular formula will usually open up, they are all known by the generic name of isomers.
Although this phenomenon is very common in organic chemistry, it is not exclusive to organic chemistry as it is also present in some inorganic compounds.
History
Isomerism was first observed in 1827: Friedrich Wöhler prepared silver cyanate (AgOCN) and noted that, despite its elemental composition being identical to that of silver fulminate (prepared by Justus von Liebig the previous year), their properties were very different. This finding challenged the prevailing chemical understanding of the time, which held that chemical compounds could be different only when they had different elemental compositions. After more discoveries of the same type, such as Wöhler's 1828 discovery that urea had the same atomic structure as the chemically different ammonium cyanate, Jöns Jakob Berzelius introduced the term isomerism in 1830 to describe this phenomenon.
In 1848, Louis Pasteur separated tiny crystals of tartaric acid into its two mirror forms. The individual molecules of each were the left and right optical stereoisomers, solutions of which rotated the plane of polarized light by the same degree, but in opposite directions.
Types of isomerism
There are two basic types of isomerism: structural and spatial.
Constitutional or structural isomerism
It is a form of isomerism, where compounds with the same molecular formula have a different distribution of bonds between their atoms, contrary to what occurs in stereoisomerism.
Due to this, three different modes of isomerism can be presented:
- Chain/skeleton.- The isomers of this type have components of the chain accommodated in different places, that is, the carbonated chains are different, have different skeleton or structure.
An example is pentane, of which there are several isomers, but the best known are isopentane and neopentane.
- I'd be in position..- It is that of those compounds in which their functional groups are united in different positions.
A simple example of this type of isomerism is that of pentanol, where there are three positional isomers: 1-pentanol, 2-pentanol and 3-pentanol.
- Functional group Isomeria.- Here, the different connectivity of atoms, can generate different functional groups in the chain. An example is cyclohexane and 1-hexeno, which have the same molecular formula (C6H12), but the cyclexane is a cyclic or cyclalcan alcano and the 1-hexeno is an alcheno.
There are several examples of isomerism such as ionization, coordination, bonding, geometry, and optics.
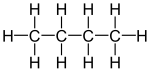
String isomerism or ordering
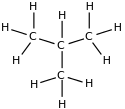
The arrangement of the carbon atoms in the chain or carbon skeleton varies, that is, its structure, which can be linear or have different ramifications depends on its length.
For example, C4H10 corresponds to both butane and methylpropane (isobutane or tert-butane):
 |  |
|---|---|
| Butano n-butan | Metilpropain iso-butano or terc-butano |
For the formula C5H12, we have three possible chain isomers: pentane, methylbutane (isopentane) and dimethylpropane (neopentane). The number of chain isomers increases rapidly with increasing number of carbon atoms.
Position isomerism
It is presented by those compounds that have the same carbon skeleton but in which the functional group occupies a different position.
For example, the molecular formula C4H10O can correspond to two isomeric substances that differ in the position of the OH group: 1-butanol.
| CH3-CH2-CH2-CH2OH | CH3-CH2-CHOH-CH3 |
|---|---|
| 1-butanol, butan-1-ol or n-butanol | 2-butanol, butan-2-ol or sec-butanol |
 | 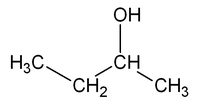 |
This type of isomerism results from the possibility of placing functional groups, side chains in structurally non-equivalent positions. Suppose we substitute one of the butane's hydrogen atoms, CH3-CH2-CH2-CH3, for a hydroxyl group. By numbering the carbons of the butane chain and making this substitution at the extreme carbon (C1), we obtain an alcohol called butan-1-ol (1-butanol). If we substitute a C2 hydrogen for the -OH group, we obtain the isomeric alcohol butan-2-ol (2-butanol), which differs in the position of the hydroxyl group. Note that, however, if we make the substitution at C3, we do not get a third isomer, but again 2-butanol. The two representations indicated for 2-butanol are structurally identical, as can be seen by rotating its structure 180º around an axis.
Compensation or compensation isomerism
Compounds in which a function cuts the carbon chain into portions of different lengths are sometimes called compensatory isomerism or metamerism.
For example, two metamers with molecular formula C4O2H8 are:
| HCOO-CH2-CH2-CH3 | CH3-COO-CH2-CH3 |
|---|---|
| Propyl methane | Ethyl ethanate |
This type of isomerism was more used in the past than it is today. It was even applied to aldehyde-ketones, which today are usually considered functional isomers.
Functional isomerism
Vary the functional group, preserving the carbon skeleton.
For example, C3H6O can correspond to the propanal molecule (aldehyde function) or propanone (ketone function).
| CH3-CH2- Right. | CH3-CO-CH3. |
|---|---|
| Propanal (aldehyde function) | Propanona (brown function) |
This isomerism is presented by certain groups of related compounds such as: Alkenes and Cycloalkanes, Alkynes, Cycloalkenes and Alkadienes, alcohols and ethers, acids and esters, and also aldehydes and ketones.
Tautomerism
It is a special type of isomerism in which there is a rearrangement of an atom between the two structures, generally hydrogen, and there is also an easy balance between both tautomeric forms.
An example of it is the keto-enol tautomerism in which there is an equilibrium between a compound with an OH group attached to one of the carbon atoms of a C=C double bond, and a compound with the intermediate carbonyl group, C=O typical of ketones, with rearrangement of a hydrogen atom.
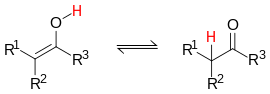 |
|---|
| Ceto-Enlic Tautoty. |
Spatial isomerism or stereoisomerism
Present stereoisomerism those compounds that have identical molecular formulas and their atoms have the same distribution (the same shape of the chain; the same functional groups and substituents; located in the same position), but their arrangement in space is different, that is, they differ in the spatial orientation of their atoms.
Stereoisomers have the same shape if they are represented in a plane. It is necessary to represent them in space to visualize the differences. They can be of two types: conformational isomerism and configurational isomerism, depending on whether the isomers can be converted into one another by simple rotation of single bonds or not.
Another classification divides them into enantiomers and diastereoisomers. Diastereoisomers include cis-trans isomers (formerly known as geometric isomers), conformers or conformational isomers, and, in molecules with multiple chiral centers, isomers belonging to different pairs of enantiomers.
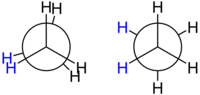
Conformational Isomerism
This type of conformational isomers or conformers, the conversion from one form to another is possible because the rotation around the axis of the bond formed by the carbon atoms is more or less free (see animation on the right). That is why they are also called rotamers.
Conformational isomers are generally not separable or isolable, due to the ease of interconversion even at relatively low temperatures. The branch of stereochemistry that studies conformational isomers that are indeed isolable (most are biphenyl derivatives) is called atropisomerism.
These shapes are easy to recognize using the Newman projection, as seen in the drawings on the left. They are called synclinal (sometimes gauche), anticline (anti or trans), synperiplanar, and antiperiplanar.
| Newmann's projections for the ethank molecule. Eclipsed and alternate forms. | Different forms of ethank according to rotation around the axis that form the two carbon atoms. Eclipsed forms or staggered forms are observed. |
Another type of conformational isomers occurs in compounds with hexagonal rings such as cyclohexane, where the chair-shaped conformation and boat-shaped conformation are feasible.
Configurational isomerism
A simple rotation is not enough to convert one form into another and even if the spatial arrangement is the same, the isomers are not interconvertible. It is divided into: geometric isomerism or cis-trans, and optical isomerism. Configurational isomers are isolable, since a large amount of energy is required to interconvert them (energy required for bond breaking).
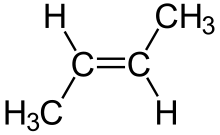
Geometric or cis-trans isomerism
cis-trans isometry is a type of stereoisomerism, which refers to compounds that have their atoms connected in the same order but have different three-dimensional orientation. This type of isomerism occurs in cycloalkanes (a series of carbons that make up a ring) and in alkenes (a chain of carbons joined by a double bond). It occurs when there are two identical groups to compare and they are in different positions.
In the case of cycloalkanes, it is named cis when the functional groups are on the same side of the ring and trans if they are on opposite sides. As can be seen in the image.
In the case of double bonds, use cis if the groups are on the same side of the double bond and trans if the groups are on opposite sides. We can see the two cases in the following images.
This type of isomerism gives different physical properties, because double bonds do not rotate freely like single ones.
It occurs when there are two carbons joined with a double bond that have the other valences with the same substituents (2 pairs) or with two equal ones and one different one.
There is no geometric isomerism linked to triple or single bonds.
The two possibilities are called:
- shape cis (or form Z), with the two most voluminous substitutes on the same side, and
- shape trans (or form E), with the two most voluminous substitutes in opposite positions (Rules of Cahn-Ingold-Prelog).
These two forms cannot be interconverted with each other spontaneously, since the double bond prevents rotation, although they can sometimes become catalyzed reactions.
| But-2-enomers | Male acid (Cis) and fumáric acid (trans) | Trans (E) and cis (Z) forms 1,2-dibromoetene. |
|---|---|---|
 |  |  |
Geometric isomerism also occurs in compounds with N=N double bonds, or in cyclic compounds in which rotation around an axis is also prevented.
| 1,2-dimethylcyclopentan (forms) cis and trans) | cis-1,2-dichlorocyclohexane | trans-1,2-dichlorocyclohexane | Cis and trans forms of difluorodiazeno |
|---|---|---|---|
 |  |  |  |
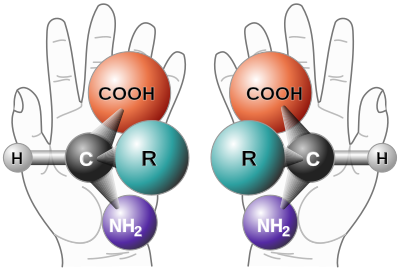
Optical isomerism or enantiomerism
When a compound has at least one asymmetric or chiral Carbon atom, that is, a carbon atom with four different substituents, two different varieties can be formed called optical stereoisomers, enantiomers, enantiomorphic forms, or chiral forms, although all atoms They are in the same position and linked in the same way. This is known as the Le Bel-van't Hoff rule.
Optical isomers cannot overlap, as they can with the right and left hands. They present the same physical and chemical properties but differ in that they deviate the plane of vibration of polarized light in a different direction:
- an isommer diverts the polarized light to the right (in orientation with the watch handles) and is represented with the sign (+): it is the dextrogic isommer or dextro form;
- the other optical isomer deviates it to the left (in orientation contrary to the watch handles) and is represented with the sign (-)(levo or levo-shaped isomer).
Another way to name these compounds is by the DL convention or nomenclature, usually using the Fischer projection. This nomenclature is absolute but the (D) form does not necessarily coincide with the dextrorotatory isomer or (+) form.
If a molecule has n asymmetric carbon atoms, it will have a total of 2n optical isomers.
These isomers can also be represented by the letters (R) and (S). This R-S nomenclature, which follows the Cahn-Ingold-Prelog rules, is also used to determine the absolute configuration of chiral carbons.
So, there are three systems of naming these compounds:
- according to the direction of deviation of the plane of the polarized light, we distinguish the dextro forms (+) and levo (-);
- according to the nomenclature D-L (Formas D and L), which is unequivocal for isomers with a single asymmetric carbon, and
- according to the absolute configuration R-S (forms R and S), more suitable for molecules with several asymmetric centers.
Diastereoisomers
When a compound has more than one asymmetric carbon, we can find enantiomeric forms and other forms that are not exactly mirror copies, because they do not have all their carbons inverted. These forms are called diastereoisomers. For example, 3-bromo-butan-2-ol has two asymmetric carbons, so it has 4 possible forms. Some of them are enantiomorphic, such as (2S,3S)-3-bromo-butan-2-ol and (2R,3R)-3-bromo-butan-2-ol. Instead, (2R,3S)-3-bromo-butan-2-ol is a diastereoisomer of the previous two.
Racemic mixture and meso forms
A racemic mixture is the equimolar mixture of the dextro and levo isomers. This formula is optically inactive (it does not bend the plane of polarized light). The mixture of D-lactic and L-lactic acid forms an optically inactive, racemic mixture.
If a compound has two asymmetric carbons, it can have one right-handed and one left-handed, but if it has a plane of symmetry, together it behaves optically inactive and is called the meso form. This is the case of tartaric or 2,3-dihydroxybutanedioic acid, one of whose isomers is a meso form.
Specific rotary power
It is the deviation suffered by the plane of polarization when polarized light passes through a solution with a concentration of 1 gram of substance per cm³ in a container 1 dm high. It is the same for both enantiomers, although with the opposite sign. It is measured with the polarimeter.
Isomerism in inorganic chemistry
There are several types of isomerism present in inorganic compounds, especially in coordination complexes, but this phenomenon is not as important as in organic chemistry:
- Structural or topological: Atoms unite differently, as in the S2F2of which there is a chain-shaped molecule and another in the form of a triangular pyramid. A special case is tautomeria, in which an H atom changes position.
- I'd think so.: Same as the one already mentioned for organic compounds. It is presented in compounds with simple link as P2H4 or ion ditionito, S2O42-, where there are eclipsed, staggered and sinclinal forms (gauche).
- I'd have cis-trans (geometric): Appears in compounds such as nitrous acid, HNO2, or in flat-screen coordination complexes like [PtCl2(NH3)2].
- I'd be in position.like in some sulfur and nitrogen heterocycles. In the S6(NH)2 the octagonal ring of sulfur is maintained but two sulfur atoms have been replaced by NH groups, which may be in position 1.2; 1.3; 1.4 and 1.5.
- I'd think it would be optical.: also appears in tetrahedical structure coordination compounds with different substitutes.
- Isomería de ionización: A ligand of the cation is exchanged with one of the anions that neutralize it, as is the case between [CrSO4(NH3)5]Cl and [CrCl(NH3)5THAT4
- Isomed of coordination: If both ions are complex, we can exchange their ligands and get different isomers, as is the case between [Co(NH)3)6][Cr(CN)6] [Cr(NH3)6][Co(CN)6].
- Isomeria liaison: Some ligands can unite differently to the central ion, as it occurs in [CoCl(NO2(NH)3)4]+ [CoCl(ONO)(NH)3)4]+
- Isomería de polimerización: This is the case of NO2 and N2O4Two gaseous nitrogen oxides.
Contenido relacionado
Barium
Halogens
Deoxyribose