Iridium
iridium is a chemical element with atomic number 77 that is located in group 9 of the periodic table. Its symbol is Go. It is a hard, brittle, heavy, silvery-white transition metal from the platinum group. It is the second densest element (after osmium) and is the most resistant element to corrosion, even at temperatures as high as 2000 °C. Only some halogens and molten salts are corrosive to iridium in the solid state. Powdered iridium is much more reactive and can become flammable.
It was discovered in 1803 among the insoluble impurities of natural platinum. Smithson Tennant, the first discoverer, named the metal iridium after the goddess Iris, the personification of the rainbow, because of the diverse and striking colors of its salts. Iridium is one of the rarest elements in the earth's crust, with an annual extraction and consumption of only three tons. 191Ir and 193Ir are the two natural isotopes of iridium and also its only stable isotopes; the 193Ir is the more abundant of the two.
The most important iridium compounds are the salts and acids that it forms together with chlorine, although iridium also forms a series of organometallic compounds, used in industrial catalysis and in research. Metallic iridium is used where high resistance to corrosion at high temperatures is needed, such as in high-end spark plugs, crucibles for high-temperature recrystallization of semiconductors, and electrodes for the production of chlorine through the process. of chloralkali. Iridium radioisotopes are used in some radioisotope generators.
Iridium is found in meteorites in much greater abundance than in the Earth's crust. For this reason, the unusually high abundance of iridium in the clay layer at the Cretaceous-Paleogene boundary gave rise to the hypothesis of Álvarez de that the impact of a massive alien object caused the extinction of the dinosaurs and many other species 65 million years ago. Similarly, an iridium anomaly in core samples from the Pacific Ocean suggested the Eltanin impact of about 2.5 million years ago. millions of years.
Iridium is also used in high-strength alloys that can withstand high temperatures. It is a rare element and is found in nature in alloys with platinum and osmium. It is used in electrical contacts, devices that work at high temperatures, and as a hardening agent for platinum.
Features
Main Features
It is white in color, similar to platinum, but has a slight yellow penetration. This metal is difficult to work with, as it is very hard and brittle. It is the most corrosion-resistant metal. It is not attacked by acids, not even by aqua regia. To dissolve it, hydrochloric acid, HCl, concentrated with sodium chlorate, NaClO3 is used at high temperatures.
Iridium is commonly considered an extraterrestrial metal, as it is abundant in meteorites and rare in the Earth's crust, with only a small concentration of 0.001 ppm. It is the densest metal after osmium. It is known to be in This metal is found in the Earth's core along with iron and nickel, its most important components.
Physical properties
It belongs to the platinum group metals. Due to its hardness, brittleness, and high melting point (the ninth highest of all elements), solid iridium is difficult to shape or work with as you would other metals, so it is preferred to work in powdered form. metallic. It is the only metal that maintains good mechanical properties above 1,600 °C. Iridium has a very high boiling point (10th among all elements) and becomes a superconductor at temperatures below 0.14 K.
Iridium's modulus of elasticity is the second highest of all elements, surpassed only by osmium; this, together with a high modulus of rigidity and low Poisson's ratio, indicates the high degree of rigidity and resistance to deformation that have made handling a matter of great difficulty. Despite these limitations and the high cost of iridium, it is very valuable for applications where mechanical resistance is an essential factor and is used in some modern technologies that operate in extreme conditions.
The measured density of iridium is slightly lower (0.1%) than that of osmium, which is the densest element known. Previously there was ambiguity as to which element was denser, due to the small difference of densities between these two elements and the difficulty to accurately measure this difference..59 g/cm³ for osmium.
Chemical Properties
Iridium is the most corrosion resistant metal known: it is not attacked by almost any acid, aqua regia (although pulverized), molten metals or silicates at high temperatures. It can, however, be attacked by some molten salts, such as sodium cyanide and potassium cyanide, as well as oxygen and halogens (particularly fluorine) at high temperatures.
Compounds
| oxidation States | |
|---|---|
| −3 | Ir(CO)3]-3 |
| −1 | [Ir(CO)3(PPh3)]- |
| 0 | Go4(CO)12 |
| +1 | [Ir(CO)Cl(PPh)3)2] |
| +2 | IrCl2 |
| +3 | IrCl3 |
| +4 | Go2 |
| +5 | Go4F20 |
| +6 | IrF6 |
Iridium forms compounds in oxidation states from -3 to +6, the most common being +3 and +4. Higher oxidation states are rare, but include IrF6 and two mixed oxides, Sr2MgIrO6 and Mr2CaIrO6. Iridium dioxide, a brown powder, is the only well-characterized oxide of iridium, an iridium sesquioxide, Ir2O3, has been described as a powder blue-black in color which oxidizes to IrO2 on exposure to HNO3. Compounds of iridium and sulfur have also been found, such as IrS3 . Iridium also forms compounds with oxidation states +4 and +5, such as K2IrO3 and KIrO3, which can be prepared from the reaction of potassium oxide or potassium superoxide with iridium at high temperatures. Currently no binary hydrides of iridium (IrxHy) are known, but complex hydrides such as IrH-4 are known. 5 and IrH-36, where iridium has oxidation number +1 and +3 respectively. The ternary hydride Mg6Ir2H11 are believed to contain both the anion IrH-45 and to IrH-36. No monohalide or dihalide of iridium is known, however, trihalides (IrX3) are known. of iridium with all halogens. For oxidation states +4 and higher, only tetrafluoride, pentafluoride, and hexafluoride are known. Iridium hexafluoride is a highly reactive, volatile yellow solid composed of octahedral molecules. It decomposes in water and is reduced to IrF4, a crystalline solid of black iridium. Iridium pentafluoride has similar properties but is actually a tetramer, Ir4F 20, formed by four octahedrons that share corners.
H2IrCl6, and its ammonium salt are the most industrially important iridium compounds. These compounds are involved in the purification of iridium and are used as precursors for most other iridium compounds, as well as in the preparation of coatings for anodes. The IrCl-26 ion has a deep dark brown color, and can be easily reduced to IrCl-36 >, lighter in color, and vice versa. Iridium trichloride (IrCl3), which can be obtained in anhydrous form from the direct oxidation of iridium powder by chlorine at 650 °C, or in hydrated form by dissolving Ir2O3 in hydrochloric acid, it is often used as a starting material for the synthesis of other Ir(III) compounds. Another compound that is used to synthesize other Ir(III) compounds is ammonium hexachloroiridium ((NH4)3IrCl6). Ir(III) compounds are diamagnetic with an octahedral molecular geometry.
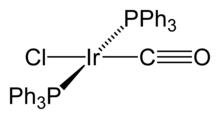
Organoiridic compounds contain iridium-carbon bonds, where the metal is generally found in the lowest oxidation states, for example, oxidation state 0 is found in dodecarboline tetrairidium (Ir4(CO)12), which is the most common and stable binary carbonyl of iridium, in this compound, each of the iridium atoms bonds to the other three, thus forming a tetrahedral structure. Some organometallic Ir(I) compounds are important enough to be named after their discoverers. One of them is the Vaska complex (IrCl(CO)[P(C6H5)3]2), which has the rare quality of binding to the diatomic oxygen molecule (O2). Another is Crabtree catalysis, a homogeneous catalysis carried out by hydrogenation reactions. These compounds have a planar quaternary structure, d8 complex, with a total of 16 valence electrons, which explains their reactivity.
Isotopes
Iridium has two stable natural isotopes, 191Ir and 193Ir, with a natural abundance of 37.3% and 62.7%, respectively. At At least 34 radioisotopes have been synthesized ranging from mass numbers 164 to 199. 192Ir, which decays into the two stable isotopes, is the most stable radioisotope with a half-life of 73,827 days. Three other isotopes, 188Ir, 189Ir, 190Ir, have a half-life of at least one day. Mass number isotopes below 191 they decay by a combination of ß-decay, α-decay, and proton emission, with the exception of 189Ir, which decays by electron capture, and 190 Ir, which decays through positron emission. Synthetic isotopes with an atomic mass greater than 191 decay by β– decay, although 192Ir can also decay to a lesser extent by electron capture. All known isotopes of iridium were discovered between 1934 and 2001., the most recent of which is 171Ir.
At least 32 metastable isomers have been characterized, ranging in atomic mass from 164 to 197, the most stable of all these is 192m2Ir, which decays by isomeric transition with a half-life of 241 years, making it more stable than any of the synthetic isotopes of iridium in its ground states. The least stable is 190m3Ir, with a half-life of just 2 µs. The isotope 191Ir was the first element to see the Mößbauer effect, making it makes Mössbauer spectroscopy useful in physical, chemical, biochemical, metallurgical, and mineralogical investigations.
History
The discovery of iridium dates from the same time that platinum and other metals in its group were discovered. Elemental platinum was used by the ancient Ethiopians and by South American cultures, which always had access to a small amount of platinum group metals, including iridium. Platinum arrived in Europe under the name of "platinum" (small silver), discovered in the XVII century by Spaniards in the region now known as the Department of Chocó in Colombia. But the discovery that this metal was a new element and not an alloy of known elements did not come until 1748.
Chemists who studied platinum found that it dissolved in aqua regia, creating soluble salts. These chemists always noted a small amount of an insoluble dark-colored residue. Joseph Louis Proust thought this residue was due to graphite. French chemists Victor Collet-Descotils, Antoine François, comte de Fourcroy, and Louis Nicolas Vauquelin also noted they observed the dark residue in 1803, however, they did not obtain enough to carry out experiments. That same year a British scientist, Smithson Tennant, analyzed the insoluble residue and concluded that it must contain a new metal. Vauquelin exposed the powdery residue to alkalis and acids and obtained a new volatile oxide, which he believed to be the new metal he called ptene, which came from the Greek word πτηνος (ptènos) and it meant "Winged". Tennant, who had a much larger amount of the residue, continued his research and identified two new elements within the black residue, iridium and osmium. He obtained colored crystals dark red (probably from Na2[IrCl6]•nH2O) by a series of reactions with sodium hydroxide and hydrochloric acid. He named one of the elements iridium after the Greek goddess Iris, after the colors of its salts. The discovery of the new elements was documented in a letter to the Royal Society on June 21, 1804.
British scientist John George Children was the first to melt a sample of iridium in 1813 with the help of the "best galvanic battery ever built" (until that time).
The first to obtain pure iridium was Robert Hare in 1842. He found that the density of iridium was around 21.8 g/cm³ and noted that the metal was not malleable and extremely hard.
The first smelting of a significant amount of the metal was carried out by Henri Sainte-Claire Deville and Jules Henri Debray in 1860. To melt the metal, it took more than 300 liters of pure O2 and H2 for every kilogram of iridium. These extreme difficulties in melting the metal have limited the possibilities of handling iridium.
John Isaac Hawkins was looking for a pen with a fine, hard nib, and in 1834 he succeeded in creating a gold pen with an iridium tip.
In 1880, John Holland and William Lofland Dudley, succeeded in melting iridium by adding phosphorus, they would later patent the process in the United States. The British company Johnson Matthey later stated that it had been using a similar process since 1837 and had already presented fused iridium at a series of fairs around the world. The first use of an iridium-ruthenium alloy was made to make thermocouples by Otto Feussner in 1933. This allowed air temperatures of up to 2,000 °C to be measured.
In 1957 Rudolf Ludwig Mößbauer discovered the effect of resonance and recoil-free absorption of gamma rays in atoms of a solid sample containing only 191Ir. For this phenomenon, known like the Mößbauer Effect (which has since been observed in other nuclei such as 57Fe), Mößbauer received the Nobel Prize in Physics in 1961, just three years after publishing his discovery. .
Abundance
Iridium is one of the least abundant elements in the Earth's crust, on average only a 0.001 ppm mass fraction is found in the entire crust; gold is 40 times more abundant, platinum 10 times more, and silver and mercury about 80 times more abundant than iridium. Tellurium is as abundant as iridium. There are only three elements as little abundant as iridium: rhenium, ruthenium and rhodium; iridium is 10 times more abundant than the latter two. In contrast to its low abundance in the Earth's crust, iridium is relatively common in meteorites, with a concentration of 0.5 ppm or more.
Iridium can be found in nature as an uncombined element or in natural alloys, especially osmium-iridium alloys, these alloys can be separated into two large groups: osmyridium alloys, which are richer in osmium, and iridiosmium containing a greater amount of iridium than osmium. It is also found in nickel and copper deposits, platinum group metals are normally found in these deposits in the form of sulfides, tellurides, antimonides, and arsenides. Within the Earth's crust, iridium is found in highest concentrations in three types of geologic structure: igneous deposits, impact craters, and deposits made from one of these structures. The largest known primary iridium reserve is from the Bushveld igneous complex in South Africa, although the large copper-nickel deposits near Norilsk in Russia and the Sudbury Basin in Canada are also important sources of iridium. Small reserves of this metal have also been found in the United States. Iridium can be found in secondary deposits, combined with platinum or other platinum group metals in alluvial deposits. These types of deposits were mined by pre-Columbian cultures in the department of Chocó, even today they continue to be a source of metals from the platinum group.
Presence in the K-T borderline
The 65-million-year K-T boundary, marking the temporal border between the Cretaceous and Cenozoic periods of geologic time, was identified due to a thin layer of iridium-rich clay, the amount of this iridium layer could contain 200,000 tons of that metal. In 1980, a team led by Luis Walter Álvarez proposed an extraterrestrial origin for all this iridium found in the layer; he attributed it to an asteroid or comet impact.This theory, known as the Álvarez hypothesis, is the most widely accepted to explain the extinction of the dinosaurs. A large buried impact crater dating to 65 million years ago was identified in what is now known as the Yucatán Peninsula (the Chicxulub crater). Dewey M. McLean and other scientists argue that this iridium could have origins volcanic because the earth's core is rich in iridium, and even today, active volcanoes such as the Piton de la Fournaise ('furnace peak') on the island of Réunion continue to release iridium.
Production
| Year | Price ($/ozt) |
|---|---|
| 2001 | 415.25 |
| 2002 | 294.62 |
| 2003 | 93.02 |
| 2004 | 185.33 |
| 2005 | 169.51 |
| 2006 | 349.45 |
| 2007 | 440.00 |
Iridium is obtained commercially as a by-product of nickel and copper mining and production. Through the electrorefining of copper and nickel, noble metals such as silver, gold and platinum group metals, as well as selenium and tellurium are deposited at the bottom of the cell as anode mud, which constitutes the point of batch for extraction. In order to separate the metals, the first thing that must be done is to dissolve the clay in a solution. There are several methods, depending on the separation process and the composition of the mixture. Two widely used methods are melting with sodium peroxide and then dissolving in aqua regia, the other consists of dissolving in a mixture of chlorine and hydrochloric acid.
After it dissolves, iridium is separated from other platinum group metals by the precipitation of (NH4)2IrCl6 > or by extraction of IrCl-26 with organic amines. The first method is similar to the Tennant and Wollaston procedure used for their separation. The second method can be planned as a continuous liquid-liquid extraction and therefore more suitable for production on an industrial scale. In either case, the product is reduced through the use of hydrogen, producing the metal in powder or sponge form that can be treated with powder metallurgy techniques.
The annual production of iridium in the year 2000 was about 3 tons, which is equivalent to approximately 100,000 troy ounces (ozt). The price of iridium reached in 2007 a price of $440 per troy ounce, but the price has fluctuated considerably, as shown in the table, in 2010 the price rose to more than 750 USD/ozt, however, on average it has remained in the range of the years 2007-2009, that is to say, from $425–$460 USD/ozt. The high volatility in the prices of metals belonging to the platinum group has been attributed to supply, demand, speculation and hoarding, amplified by the small size of the market and the instability of the prices. producing countries.
Applications
Industrial and medical
The high melting point, hardness, and corrosion resistance of iridium and its alloys determine most of their applications. Iridium and especially iridium-platinum or osmium-iridium alloys tend to wear very little and are used, for example, in multiple rows of pores, through which a molten plastic is extruded to form fibers, such as rayon. Osmium-iridium alloys are used in compasses and balances.
Iridium's resistance to corrosion and heat make it an important alloying agent. Some long-life parts in aircraft engines are made of alloyed iridium, and a special titanium-iridium alloy is used in deepwater pipelines because of its resistance to corrosion. Iridium is also widely used as a hardening agent in titanium alloys. platinum. The Vickers hardness of pure platinum is 56 HV, while that of an alloy with 50% iridium can reach hardnesses above 500 HV.
Devices that are exposed to extreme temperatures are often made from iridium, for example, high-temperature crucibles made from iridium are used in the Czochralski process to produce oxide single crystals (such as sapphires) for use in memory devices in computers and solid-state lasers. The high abrasion resistance of iridium and its alloys make it ideal for making electrical contacts in spark plugs.
Iridium compounds are used as catalysts in the Cativa process for the carbonylation of methanol to produce acetic acid. Iridium itself is used as a catalyst in a type of automotive engine introduced in 1996 called a direct ignition engine. The radioisotope 192Ir is one of the two most important energy sources for industrial use of γ-ray radiography in non-destructive testing for metals. In addition, 192Ir is used as a source of gamma radiation for the treatment of cancer using brachytherapy, a form of radiation therapy where a sealed radioactive source is placed inside or next to the area requiring treatment.
Scientists
In 1889 an alloy of 90% platinum and 10% iridium was used to build the international prototype meter and kilogram made by the International Bureau of Weights and Measures near Paris. The definition of the meter bar was replaced from the fundamental unit of measurement in 1960 by a line from the atomic spectrum of krypton, but the prototype kilogram remains the international standard of mass. Iridium has been used in radioisotope thermoelectric generators for unmanned spacecraft, such as Voyager, Viking, Pioneer, Cassini, Galileo and the New Horizons spacecraft. Iridium was chosen to encapsulate the plutonium-238 fuel in the generator due to the material's high strength and its operational capabilities above 2,000 °C. Iridium is also used to generate optical X-rays, especially in telescopes. of X-rays. The mirrors of the Chandra X-ray observatory are coated with a 60 nm thick layer of iridium. Iridium proved to be the best option to reflect X-rays, surpassing metals such as nickel, gold, platinum. The iridium layer, which had to be only a few atoms thick, was applied by high vacuum depositing gaseous iridium onto a chromium base layer.
Iridium is used in particle physics for the production of antiprotons, a form of antimatter. Antiprotons are produced by shooting a high-intensity beam of protons at a conversion target, which must be made of an extremely dense material. Although tungsten can be used instead of iridium, the latter has the advantage of better stability under shock waves induced by the rise in temperature during the incident beam. Iridium complexes are being investigated as catalysts for asymmetric hydrogenation. These catalysts have been used in the synthesis of natural products capable of hydrogenating certain difficult substrates, such as alkenes, enantioselectively (the generation of only one of two possible enantiomers). Iridium forms a variety of complexes of fundamental interest in the collection of triplets.
Historical
Iridium-osmium alloys have been used in fountain pens. The first use of a significant amount of iridium was in 1834 in a gold-mounted iridium nib. From 1944, the famous Parker 51 fountain pen was fitted with a nib made of a gold alloy. ruthenium and iridium (3.8% iridium). Platinum-iridium alloys have been used in barrel vents; this is an important application as it avoids the expense of wearing out these holes when in service. The pigment "iridium black", which consists of very finely divided iridium, is used to color porcelains with a deep black color.
Precautions
Iridium in metal form is not hazardous to health due to its low tissue reactivity, there are only 20 parts per trillion iridium in human tissues. However, finely divided iridium powder can be dangerous handling, as it is an irritant and can ignite in air. Very little is known about the toxicity of iridium compounds due to the scarcity of the metal and because its compounds are used in very small amounts, but the soluble salts, such as iridium halides, could be dangerous due to the other elements that are part of the compound. However, the vast majority of iridium compounds are insoluble, making unintentional absorption of these compounds by the body difficult. A radioisotope of iridium, 192Ir, is as dangerous as any other radioactive isotope. The only reports related to iridium lesions concern accidental exposure to 192Ir used in brachytherapy. High energy gamma ray radiation from 192Ir can increase the risk of cancer. External exposure can cause burns, radiation poisoning, and death. Ingestion of 192Ir can burn the lining of the stomach and intestines. 192Ir, 192mIr and 194mIr tend to be deposited in the liver, and can pose health risks from both gamma radiation and beta radiation.
Contenido relacionado
Nordic gold
Ethylenediaminetetraacetic acid
Germanium