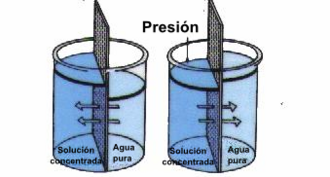
Inverse osmosis
Reverse osmosis is a water purification technology that uses a semi-permeable membrane to remove ions, molecules and larger particles in drinking water. To achieve reverse osmosis, a pressure is applied to overcome the osmotic pressure, which is a colligative property produced by differences in the chemical potential of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of suspended elements in water, including bacteria, and is used both in industrial processes and for the production of drinking water. The result is that the solution is retained on the pressurized side of the membrane and the pure solvent can pass through to the other side. To achieve "selectivity", this membrane must not let ions or large molecules pass through its pores (or holes), but must freely pass smaller components of the solution (such as solvent molecules).
In the normal process of osmosis, the solvent moves naturally from an area of low solution concentration (high water potential), through a membrane, to an area of high solution concentration (low water potential).. The force that causes the movement of the solvent is the reduction in the free energy of the system when the difference in the concentration of the solvent on either side of a membrane is reduced, generating osmotic pressure because the solvent moves into the solution more concentrated. Reverse osmosis is the application of external pressure to reverse the natural flow of the solvent. The process is similar to other applications of membrane technology. However, there are key differences between reverse osmosis and filtration. The predominant extraction mechanism in membrane filtration is size exclusion, so the process can theoretically always achieve perfect efficiency regardless of pressure and concentration. Reverse osmosis applies diffusion, making the process dependent on pressure, flow rate, and other conditions. Reverse osmosis is generally used for the purification of drinking water from seawater by extracting salt and other effluents from the water molecules.
Contenido relacionado
Ammonium
Donald glaser
Endemism