Inorganic chemistry

A: The Diborano has an unusual link
B: Cesium chloride has an archetypal crystalline structure. C: The Fp2 It's an organometallic complex. D: Silicone uses range from breast implants to Silly Putty.
E: Grubbs' catalyst won the 2005 Nobel Prize for his discoverer
F: Zeolites find extensive use as molecular tamics.
G: The copper acetate (II) surprised the theorists with their diamagnetism
Inorganic chemistry is responsible for the integrated study of the formation, composition, structure, and chemical reactions of inorganic elements and compounds (for example, sulfuric acid or calcium carbonate); that is, those that do not have carbon-hydrogen bonds, because these belong to the field of organic chemistry. This separation is not always clear, as for example in organometallic chemistry, which is a superposition of both.
Formerly it was defined as the chemistry of inorganic matter, but it became obsolete when the hypothesis of the vital force was discarded, a characteristic that was supposed to be characteristic of living matter that could not be created and allowed the creation of organic molecules.
It has applications in all fields of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, pharmaceuticals, fuels, and agriculture.
Key concepts
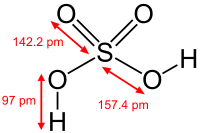
Many inorganic compounds are ionic compounds, consisting of cations and anions held together by ionic bonds. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium (Mg2+ cations) and chloride (Cl - anions ) or sodium oxide, Na2O, consisting of sodium cations, Na+, and oxygen anions, O 2− . In any salt, the proportions of the ions are such that the electrical charges cancel out, so that the compound is electrically neutral. Ions are described by their oxidation state, and their ease of formation can be inferred from the ionization potential (for cations) or electron affinity (for anions) of the parent elements.
Important classes of inorganic compounds are oxides, carbonates, sulfates, and halides. Many inorganic compounds are characterized by their high melting points. Inorganic salts are normally poor conductors in the solid state. Another important feature is its ease of crystallization. While some salts (eg NaCl) are highly soluble in water, others (eg AgCl) are not.
The simplest inorganic reaction is double displacement when, by mixing two salts, ions are exchanged without changing the oxidation state. In redox reactions, however, one reactant, the oxidant, decreases its oxidation state and another reactant, the reductor, sees its oxidation state increased. The net result is an exchange of electrons. Electron exchange can also occur indirectly, for example in batteries, a key concept in electrochemistry.
When a reactant contains hydrogen atoms, a reaction can occur by exchanging protons in acid-base chemistry. In a more general definition, any chemical species capable of binding pairs of electrons is called a Lewis acid; conversely, any molecule that tends to donate an electron pair is called a Lewis base. As a refinement of acid-base interactions, the ABDB theory also takes into account the polarizability and the size of the ions.
Inorganic compounds are found in nature as minerals. For example, the soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Inorganic compounds are also found with various functions as biomolecules: as electrolytes (sodium chloride), in energy storage (ATP), or in construction (the polyphosphate backbone in DNA).
The first important man-made inorganic compound was ammonium nitrate for soil fertilization through the Haber process. Some inorganic compounds are synthesized for use as catalysts such as vanadium(V) oxide and titanium(III) chloride, or as reagents in organic chemistry, such as lithium aluminum hydride.
Subdivisions of inorganic chemistry are organometallic chemistry, cluster chemistry, and bioinorganic chemistry. These fields are active areas of research in inorganic chemistry, directed toward new catalysts, superconductors, and therapies.
Industrial inorganic chemistry
Inorganic chemistry is a highly practical area of science. Traditionally, the scale of a nation's economy could be gauged by its production of sulfuric acid. The top 20 inorganic chemicals manufactured in Canada, China, Europe, India, Japan, and the United States (2005 data) are: aluminum sulfate, ammonia, ammonium nitrate, carbon black ammonium sulfate, chlorine, hydrochloric acid, hydrogen, hydrogen peroxide, nitric acid, nitrogen, oxygen, phosphoric acid, sodium carbonate, sodium chlorate, sodium hydroxide, sodium silicate, sodium sulfate, sulfuric acid and titanium dioxide.
The manufacture of fertilizers is another practical application of inorganic industrial chemistry.
Descriptive Inorganic Chemistry
Descriptive inorganic chemistry focuses on the classification of compounds based on their properties. In part, the classification focuses on the position in the periodic table of the heaviest element (the element with the greatest atomic weight) in the compound, in part by grouping compounds by their structural similarities. When studying inorganic compounds, they are often classified within the different parts of inorganic chemistry (an organometallic compound is characterized by its coordination chemistry and in turn can show interesting properties in the solid state).
The different classifications are:
Coordination Compounds
Classical coordination compounds include metals bound to "lone pairs" of electrons belonging to the atoms of the main group of ligands such as H2O, NH3, Cl− and CN−. In modern coordination compounds almost all organic and inorganic compounds can be used as ligands. The "metal" it normally corresponds to groups 3-13, as well as trans-lanthanides and trans-actinides, taking into account that from a certain perspective, all chemical compounds can be described as coordination complexes.
The stereochemistry of coordination complexes can be very varied, as can be seen from the Werner separation of two enantiomers of [Co((OH)2Co(NH3 >)4)3]6+, an early demonstration that chirality is not inherent only in organic compounds. A subject included within this specialization is supramolecular coordination chemistry.
- Examples: [Co(EDTA)]−[Co(NH]3)6]3+, TiCl4(THF)2.
Main Group Compounds
These compounds contain elements from groups I, II, III, IV, V, VI, VII, 0 (excluding hydrogen) of the periodic table. Due to their often similar reactivity, they can also include group 3 (Sc, Y, and La) and group 12 (Zn, Cd, and Hg) elements, as well as lanthanides and actinides.
Main group compounds have been known since the beginnings of chemistry, for example, elemental sulfur and white distillable phosphorus. Lavoisier and Priestley's experiments with oxygen, O2 not only identified an important diatomic gas, but also paved the way for describing compounds and reactions according to stoichiometric ratios. The discovery of a practical synthesis of ammonia with iron catalysts by Carl Bosch and Fritz Haber in the early 1900s had a profound impact on mankind, demonstrating the importance of inorganic chemical synthesis. Typical main group compounds are SiO2, SnCl4 and N2O. Many main group compounds can also be classified as organometallic, since they contain organic groups, for example, B(CH3)3. Main group compounds are also found in nature, for example phosphate in DNA, and can therefore be classified as bioinorganic. Conversely, organic compounds that lack (many) hydrogen ligands can be classified as inorganic, such as fullerenes, nanotubes, and binary carbon oxides.
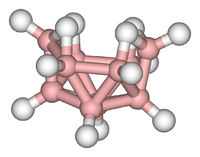
- Examples: tetranitrute sulfur S4N4Diboran B2H6, silicone, buckminsterfullerene C60.
Transition metal compounds
Compounds containing metals from groups 4 to 11 are considered transition metal compounds. Compounds with a metal from group 3 or 12 are also sometimes incorporated into this group, although they may also be classified as main group compounds.
Transition metal compounds show varied coordination chemistry, ranging from tetrahedral for titanium (eg, TiCl4) to square planar for some nickel complexes or the octahedral for cobalt coordination complexes. Some transition metals can be found in biologically important compounds, such as iron in hemoglobin.
- Examples: iron pentacarbonyl, titanium tetrachloride, cisplatin.
Organometallic compounds
Organometallic compounds are typically considered to contain the group M–C–H. The metal (M) in these species can be either a main group element or a transition metal. Operationally, the definition of an organometallic compound is more flexible, and also includes highly lipophilic complexes, such as metal carbonyls and even metal alkoxides.
Organometallic compounds are considered a special category mainly because organic ligands are often sensitive to hydrolysis or oxidation, requiring organometallic chemistry to employ more specialized preparation methods than traditional Werner-type complexes. Synthetic methods, especially the ability to handle complexes in solvents of low coordination power, allow for very weakly coordinating ligands such as hydrocarbons, H 2, and N 2. Given that these are ligands are tied to petrochemistry in a sense, organometallic chemistry has benefited greatly from its relevance to industry.
- Examples: Cyclopentadienilhierro dicarbonyl (C)5H5)Fe(CO)2CH3, Ferroceno Fe(C5H5)2, hexacarbonil molybdenum, Mo(CO)6Diborano B2H6, Tetrakis (trifenilfosfina) palate (0) Pd[P(C)6H5)3]4
Cluster (cluster) compounds
Clusters can be found in all classes of chemical compounds. According to the commonly accepted definition, a cluster consists of a set (at least triangular) of atoms that are directly linked to each other. But metal-metal bonded dimetal complexes are especially relevant in this area. Clusters occur in "pure" inorganic systems, in organometallic chemistry, main group chemistry, and bioinorganic chemistry. The distinction between very large and solid "gross" it's getting blurrier. This interface is the chemical basis of nanoscience or nanotechnology and arises specifically from the study of the effects of quantum size in cadmium selenide clusters. Therefore, large clusters can be described as a structure of bonded atoms intermediate between a molecule and a solid.
- Examples: Fe3(CO)12B10H14[Mo]6Cl14]2−4Fe-4S
Bioinorganic compounds
By definition, these compounds occur naturally, but the subfield includes anthropogenic species, such as some pollutants (for example, methylmercury) and drugs (for example, cisplatin). The field, which also encompasses many aspects of biochemistry, includes many types of compounds, for example, the phosphates in DNA, and also metal complexes that contain ligands ranging from biological macromolecules, normally peptides, to poorly defined species, such as humic acid, or water (for example, when it is present). coordinated in the gadolinium complexes used for NMR). Traditionally, bioinorganic chemistry focused on the transfer of electrons and energy in proteins relevant to respiration. Medicinal inorganic chemistry includes the study of non-essential and essential elements with applications for diagnosis and therapies.
- Examples: hemoglobin methylmercurio carboxipeptidasa.
Composites in solid state
This important area focuses on the structure, bonds, and physical properties of materials. In practice, solid-state inorganic chemistry uses techniques such as crystallography to understand the properties resulting from the collective interactions between the subunits of the solid. Within solid state chemistry are metals and their alloys or intermetallic derivatives. Related fields are condensed matter physics, mineralogy, and materials science.
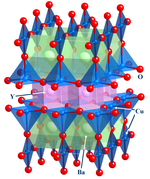
- Examples: silicone chips, zeolites, YBa2Cu3O7
Theoretical Inorganic Chemistry
An alternative perspective in the area of inorganic chemistry begins with the Bohr model of the atom and, using the tools and models of theoretical chemistry and computational chemistry, expands to bond formation in single molecules and then beyond. complex. Precise descriptions of quantum mechanics for multielectron species, which constitute the realm of inorganic chemistry, are difficult. This challenge has spawned many semi-quantitative or semi-empirical approaches including molecular orbital theory and ligand field theory. Parallel to these theoretical descriptions, approximate methodologies are employed, including density functional theory.
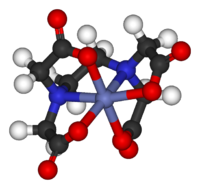
Exceptions to theories, qualitative and quantitative, are extremely important in the development of this field. For example, CuII2(OAc)4(H2O)2 is nearly diamagnetic below room temperature, while crystal field theory predicts that the molecule would have to have two unpaired electrons. The disagreement between qualitative (paramagnetic) theory and (diamagnetic) observation led to the development of models for 'magnetic coupling'. These improved models led to the development of new magnetic materials and new technologies.
Qualitative theories
Inorganic chemistry has benefited greatly from qualitative theories. Such theories are easier to learn, since they require little training in quantum theory. Within main group compounds, TRePEV theory predicts, or at least rationalizes, the structures of main group compounds, such as the explanation why NH 3 is pyramidal, while ClF 3 is T-shaped. For transition metals, crystal field theory allows one to understand the magnetism of many simple complexes, for example, why [FeIII(CN)6]3− has only one unpaired electron, while [FeIII(H2O)6]3+ has five. The qualitative approach, especially powerful for evaluating structure and reactivity, begins with classifying molecules by number of electrons, focusing on the number of valence electrons in a molecule's central atom, typically.
Group theory and molecular symmetry
A central construct of inorganic chemistry is the theory of molecular symmetry. Group theory provides the language for describing the shapes of molecules according to their point-group symmetry. Group theory also allows factoring and simplifying theoretical calculations.
Spectroscopic features are analyzed and described with respect to symmetry properties of, among others, vibrational or electronic states. Knowledge of the symmetry properties of the ground and excited states makes it possible to predict the number and intensity of absorptions in the vibrational and electronic spectra. A classical application of group theory is the prediction of the number of C-O vibrations in substituted metal carbonyl complexes. The most common applications of symmetry in spectroscopy involve vibrational and electronic spectra.
As a teaching tool, group theory highlights the commonalities and differences between the bonds of disparate species, such as WF6 and Mo(CO)6 or CO2 and NO2.
Thermodynamics and inorganic chemistry
An alternative quantitative approach to inorganic chemistry focuses on reaction energies. Although this approach is highly traditional and empirical, it is very useful. General concepts expressed in thermodynamic terms include redox potential, acidity, and phase changes. A classic concept in inorganic thermodynamics is the Born-Haber cycle, which is used to evaluate the energy of elementary processes such as electron affinity, some of which cannot be directly observed.
Mechanisms in inorganic chemistry
An important and increasingly popular aspect of inorganic chemistry focuses on reaction pathways. The reaction mechanisms are discussed differently for different classes of compounds.
Elements of the main group and lanthanides.
The mechanisms of main group compounds from groups 13-18 are usually discussed in the context of organic chemistry (organic compounds are main group compounds, after all). Elements heavier than C, N, O, and F often form compounds with more electrons than the octet rule predicts, as explained in the hypervalent molecules article. The mechanisms of their reactions differ from organic compounds for this reason. Elements lighter than carbon (B, Be, Li) as well as Al and Mg often form electron-deficient structures that are electronically similar to carbocations. These electron-deficient species tend to react via associative pathways. The chemistry of the lanthanides mirrors many aspects of the chemistry seen for aluminum.
Transition metal complexes
The mechanisms for transition metal reactions are discussed differently than for main group compounds. The important role of d orbitals in bonding strongly influences the pathways and degree of ligand substitution and dissociation. These topics are covered in articles on coordination chemistry and ligands. Both associative and dissociative pathways are seen.
A general aspect of mechanistic transition metal chemistry is the kinetic lability of the complex illustrated by the exchange of free and bound water in the prototypical [M(H22)O)6 ]n+:
- [M(H)2O)6]n+ + 6 H2O* → [M(H2O*)6]n+ + 6 H2O
- where H2O* denotes water enriched with isotopes, for example, H217O
Rates of water exchange vary by 20 orders of magnitude on the periodic table, with lanthanide complexes at one end and the slower Ir(III) species at the other.
Redox reactions
Redox reactions are prevalent in transition elements. Two classes of redox reactions are considered: atom transfer reactions, such as oxidative addition/reductive elimination, and electron transfer reactions. A fundamental redox reaction is "self-exchange," which involves the degenerate reaction between an oxidant and a reductant. For example, permanganate and manganate, its derivative by reduction in one electron, exchange one electron:
- [MnO]4]− + [Mn*O4]2− → [MnO]4]2− + [Mn*O4]−
Ligand reactions
Coordinated ligands show different reactivity than free ligands. For example, the acidity of the ammonia ligands in [Co(NH3)6]3+ is high relative to NH 3 in Yeah. Alkenes bound to metal cations are reactive towards nucleophiles, whereas alkenes are normally not. The extensive and industrially important area of catalysis is based on the ability of metals to modify the reactivity of organic ligands. Homogeneous catalysis occurs in solution and heterogeneous catalysis occurs when gaseous or dissolved substrates interact with solid surfaces. Traditionally homogeneous catalysis is considered to be part of organometallic chemistry, and heterogeneous catalysis is discussed in the context of surface science, a subfield of solid state chemistry. But the basic inorganic chemical principles are the same. Transition metals almost exclusively react with small molecules such as CO, H2 O2, and C2H4. The industrial importance of these raw materials drives the already active area of catalysis. Ligands can also undergo transfer reactions, such as transmetalation.
Characterization of inorganic compounds
Due to the wide range of elements and the corresponding properties of their derivatives, inorganic chemistry is closely associated with many methods of analysis. Older methods tended to examine general properties, such as electrical conductivity of solutions, melting points, solubility, or acidity. With the advent of quantum theory and the corresponding expansion of electronic equipment, new tools have been introduced to test the electronic properties of inorganic molecules and solids. Often these measurements provide relevant information for theoretical models. For example, measurements in the photoelectron spectrum of methane demonstrated that the predictable two-center, two-electron bond description between carbon and hydrogen using valence bond theory is not adequate for describing the ionization processes of simple way. Such contributions led to the popularization of the theory of molecular orbitals as completely delocalized orbitals and are a more appropriate simple description of the loss or excitation of electrons.
The most common techniques are:
- X-ray crystallography: This technique allows the determination in 3D of molecular structures.
- Dual polarization interferometer: this technique measures the conformation and conformational change of molecules.
- Several forms of spectroscopy.
- Ultraviolet-visible spectrooscopy: historically, this has been an important tool, as many inorganic compounds are heavily colored
- MRI Spectroscopy: in addition to 1H and 13C, many other "good" cores for MRI (e.g. 11B, 19F 31P 195Pt) provide important information on the properties and structure of the compound. Also the MRI of paramagnetic species can result in important structural information. Proton MRI is also important because the light core of hydrogen is not easily detected by X-ray crystallography.
- Infrared spectroscopy: mainly for carbonyl ligand absorptions.
- Dual electronic nuclear resonance imaging (ENDOR)
- Mössbauer Spectroscopy
- Electronic spinal resonance: ESR (or EPR) allows the measurement of the environment of paramagnetic metallic centers.
- Electrochemical: cyclic voltametry and related techniques detect the rédox characteristics of the compounds.
Inorganic synthesis chemistry
Although some inorganic species can be obtained in pure form from nature, most are synthesized in chemical plants and in the laboratory.
Inorganic synthesis methods can be classified according to the volatility or solubility of the component reagents. Soluble inorganic compounds are prepared using organic synthesis methods. For compounds containing air-reactive metals, the Schlenk line and glove box techniques are followed. Volatiles and gases are handled in "vacuum manifolds," consisting of glass tubing interconnected through valves, all of which can be brought to a vacuum of 0.001 mm Hg or less. The compounds are condensed using liquid nitrogen (teb. 78 K) or other cryogens. Solids are typically prepared using tube furnaces, with reagents and products sealed in containers, often of fused silica (SiO2 amorphous), or sometimes more specialized materials, such such as welded Ta tubes or Pt boats. Products and reactants are transported between temperature zones to drive reactions.
Classification of inorganic compounds
Inorganic compounds are divided according to their structure into: <references>
Binary Compounds
- Metallic oxides
- Non-metallic or anhydrated oxides
Ternary compounds
- Hydroxides
- Oxoacids
- Neutral oxygen
Quaternary compounds
- Oxisal acids
Areas of interest
Sections of interest in inorganic chemistry include:
- The periodic table of the elements:
- Representative elements
- Transition metals
- Rare lands
- Coordination chemistry
- Chemistry of compounds with metal-metal link
Related areas
Areas of overlap with other fields of knowledge include:
- Materials science
- Geochemistry
- Magnetochemical
- Mining
- Analytical chemistry
- Bioinorganic chemistry
- Chemical solid state
- Physical
- Environmental chemistry
- Organizational chemistry
Important compounds and substances
There are many inorganic compounds and substances of great commercial and biological importance. Among them:
- many fertilizers, such as amonic nitrate, potassium, phosphates or sulfates...
- many daily substances and solvents, such as ammonia, oxygenated water, bleach, salfuman...
- many atmospheric gases, such as oxygen, nitrogen, carbon dioxide, nitrogen and sulfur oxides...
- all metals and alloys
- windows, bottles, televisions...
- ceramics of domestic, industrial utensils, or the tiles of the space shuttles.
- the calcium carbonate of our bones
- semiconductor silicon chips that make microelectronics and computers possible
- LCD displays
- fiber optic cable
- many industrial-interest catalysts
- the active center of metaloenzymes
See also
- IUPAC
- Nomenclature
- Chemical nomenclature of inorganic compounds
- Chemical nomenclature of organic compounds
Contenido relacionado
Disaccharide
Organic chemistry
Silicon