Infrared radiation
Infrared radiation, or IR radiation is a type of electromagnetic radiation, with a longer wavelength than visible light, but shorter than that of microwaves. Therefore, it has a lower frequency than visible light and higher than microwaves. Its wavelength ranges from about 0.7 to 1000 micrometers. Infrared radiation is emitted by any body whose temperature is greater than 0 Kelvin, that is, −273.15 degrees Celsius (absolute zero).
Therefore, it is invisible to the human eye. IR is generally understood to span wavelengths from the nominal red edge of the visible spectrum, around 700 nanometers (frequency 430 THz), down to 1 millimeter (300 GHz) (although longer IR wavelengths are more often referred to as terahertz radiation). Blackbody radiation from objects near room temperature is almost entirely of infrared wavelength. As a form of electromagnetic radiation, infrared radiation propagates energy and momentum, with properties that correspond to the wave-particle duality of a wave and a particle, the photon.
Infrared radiation was discovered in 1800 by astronomer Sir William Herschel, who discovered a type of invisible radiation in the lower energy spectrum than red light, through its effect on a thermometer. Eventually it was found that slightly more than Half of the total energy from the Sun reaches Earth in the form of infrared. The balance between absorbed and emitted infrared radiation has a critical effect on Earth's climate.
Infrared radiation is emitted or absorbed by molecules when they change their rotational-vibrational motions. It excites vibrational modes in a molecule through a change in dipole moment, making it a useful range of frequencies for studying these energy states for molecules of the proper symmetry. Infrared Spectroscopy examines the absorption and transmission of photons in the infrared range.
Infrared radiation is used in industrial, scientific, military, commercial, and medical applications. Night vision devices that use near-infrared active illumination allow people or animals to be observed without the observer being detected. Infrared astronomy uses telescopes equipped with sensors to penetrate dusty regions of space such as molecular clouds, to detect objects such as planets, and to view highly redshifted objects from the early universe. Infrared thermal imaging cameras are used to detect heat loss in isolated systems, to observe changes in blood flow in the skin, and to detect overheating of electrical components.
Military and civilian applications include target acquisition, surveillance, night vision, homing, and tracking. Human beings, at normal body temperature, radiate mainly at wavelengths of about 10 μm (micrometers). Non-military uses include thermal efficiency analysis, environmental monitoring, industrial facility inspection, growing operation detection, remote temperature sensing, short-range wireless communication, spectroscopy, and weather forecasting.
History
Infrared was discovered in the 1800s by William Herschel, an English astronomer of German origin. Herschel placed a mercury thermometer in the spectrum obtained by a glass prism in order to measure the heat emitted by each color. He discovered that the heat was strongest on the red side of the spectrum and observed that there was no light there. This is the first experience showing that heat can be transmitted by an invisible form of light. Herschel called this radiation "heat rays", a name quite popular throughout the XIX century, which, finally, it was giving way to the most modern infrared radiation.
The first infrared radiation detectors were bolometers, instruments that capture radiation by the increase in temperature produced in an absorbing detector.
Definition and relationship with the electromagnetic spectrum
Infrared radiation extends from the nominal red edge of the visible spectrum at 700 nanometers (nm) down to 1 millimeter (mm). This range of wavelengths corresponds to a frequency range of approximately 430 THz up to 300 GHz. Below the infrared is the microwave portion of the electromagnetic spectrum.
| Comparison of light | |||||||
| Name | Wave length | Frequency (Hz) | Fotonic energy (eV) | ||||
|---|---|---|---|---|---|---|---|
| Gamma rays | less than 0.01 nm | more than 30 EHz | more than 124 keV | ||||
| X-ray | 0.01 nm - 10 nm | 30 EHz - 30 PHz | 124 keV - 124 eV | ||||
| Ultraviolet | 10 nm - 400 nm | 30 PHz - 790 THz | 124 eV - 3.3 eV | ||||
| Visible light | 400 nm - 700 nm | 790 THz - 430 THz | 3.3 eV – 1.7 eV | ||||
| Infrared | 700 nm - 1 mm | 430 THz - 300 GHz | 1.7 eV – 1.24 meV | ||||
| Microwave | 1 mm - 1 m | 300 GHz – 300 MHz | 1.24 meV - 1.24 μeV | ||||
| Radio | 1 m - 10,000 km | 300 MHz - 30 Hz | 1.24 μeV - 124 feV | ||||
Natural infrared
Sunlight, at an effective temperature of 5,780 kelvins, (5,510 °C, 9,940 °F), consists of a near-thermal spectrum radiation that is slightly more than half that of infrared. At the zenith, sunlight provides an irradiance of just over 1 kilowatt per square meter at sea level. Of this energy, 527 watts are from infrared radiation, 445 watts from visible light, and 32 watts from ultraviolet radiation. Almost all of the infrared radiation in sunlight is near-infrared, less than 4 micrometers.
At the Earth's surface, at temperatures much lower than the Sun's surface, part of the thermal radiation consists of infrared in the mid-infrared region, much longer than in sunlight. However, blackbody, or thermal, radiation is continuous: it emits radiation at all wavelengths. Of these natural processes of thermal radiation, only lightning and natural fires are hot enough to produce much visible energy, and fires produce much more infrared energy than visible light.
Classification
Infrared are classified, according to their wavelength, as follows:
- near infrared from 0.7 to 1.0 μm (from the approximate end of the human eye response to silicon).
- Shortwave infrared: 1.0 to 3 μm (from silicon cut to the MWIR atmospheric window). InGaAs covers up to about 1.8 μm; less sensitive lead salts cover this region.
- medium infrared 3 to 5 μm (defined by the atmospheric window and covered by Indian antimonide [InSb] and cadmium mercury (HgCdTe) and partly by PbSe lead seleniide).
- Long wavelengths: 8 to 12 or 7 to 14 μm (this is the atmospheric window covered by HgCdTe and microballometers).
- Very long wavelength (VLWIR) (from 12 to approximately 30 μm, covered by doped silicon).
- distant infrared (from 50 μm to 1000 μm)
Near infrared is the region closest in wavelength to radiation detectable by the human eye. the mid and far infrared progressively move away from the visible spectrum. Other definitions follow different physical mechanisms (emission peaks, vs. bands, water absorption) and newer ones follow technical reasons (common silicon detectors are sensitive at about 1,050 nm, while InGaAs sensitivity starts around 950 nm). and ends between 1700 and 2600). nm, depending on the specific configuration). No international standards are currently available for these specifications.
Infrared occurrence is defined (according to different standards) at various values, typically between 700 nm and 800 nm, but the boundary between visible and infrared light is not precisely defined. The human eye is notably less sensitive to light above 700 nm in wavelength, so the longer wavelengths make negligible contributions to scenes illuminated by common light sources. However, particularly intense near-infrared light (eg, from IR lasers, IR LED sources, or from bright daylight with visible light removed by colored gels) can be detected down to about 780 nm and will be detected. will perceive as red light. Intense light sources providing wavelengths up to 1050 nm can be seen as a dull red glow, causing some difficulty in near-infrared illumination of scenes in the dark (this practical problem is usually solved by indirect illumination).). Leaves are particularly bright in the near infrared, and if all visible light leaks around an IR filter are blocked and the eye has a moment to adjust to the extremely dim image coming from an IR-pass photographic filter visually opaque, It is possible to see the Wood effect consisting of foliage glowing in infrared. [twenty]
Matter, due to its energetic characterization (see black body) emits thermal radiation. In general, the wavelength where a body emits the maximum radiation is inversely proportional to its temperature (Wien's Law). In this way, most objects at everyday temperatures have their maximum emission in the infrared. Living things, especially mammals, emit a large proportion of radiation in the infrared part of the spectrum, due to their body heat.
The power emitted in the form of heat by a human body, for example, can be obtained from the surface of its skin (about 2 square meters) and its body temperature (about 37 °C, that is 310 K), via the Stefan-Boltzmann Law, and turns out to be around 100 watts.
This is closely related to the so-called "thermal sensation", according to which we can feel hot or cold regardless of the ambient temperature, depending on the radiation we receive (for example from the Sun or other hot bodies nearest): If we receive more than the 100 watts that we emit, we will be hot, and if we receive less, we will be cold. In both cases the temperature of our body is constant (37 °C) and that of the air that surrounds us as well. Therefore, the thermal sensation in still air only has to do with the amount of radiation (usually infrared) that we receive and its balance with what we constantly emit as hot bodies that we are. If, on the other hand, there is wind, the layer of air in contact with our skin can be replaced by air at a different temperature, which also alters the thermal balance and modifies the thermal sensation.
Regions within the infrared
In general, objects emit infrared radiation across a spectrum of wavelengths, but sometimes only a limited region of the spectrum is of interest because sensors typically pick up radiation only within a specific bandwidth. Thermal infrared radiation also has a maximum emission wavelength, which is inversely proportional to the absolute temperature of the object, according to Wien's displacement law. The infrared band is often subdivided into smaller sections, although the way the infrared spectrum is divided varies depending on the different areas in which infrared is used.
Visible Boundary
Infrared, as its name implies, is generally considered to begin at wavelengths longer than those visible to the human eye. However, there is no "hard" of wavelength for what is visible, since the sensitivity of the eye decreases rapidly but gently, for wavelengths greater than about 700 nm. Therefore, the longer wavelengths can be seen if they are bright enough, although they can still be classified as infrared by the usual definitions. Thus, the light from a near-infrared laser can appear dim red in color and can pose a hazard as it can be quite bright. And even infrared with wavelengths up to 1,050 nm from pulsed lasers can be seen by humans under certain conditions.
Commonly used subdivision scheme
A commonly used subdivision scheme is:
| Name | Abbreviation | Wave length | Frequency | Fotonic energy | Temperature | Features |
|---|---|---|---|---|---|---|
| Nearby infrequency | NIR, IR-A DIN | 0.75-1.4 μm | 214–400 THz | 886–1,653 meV | 3,864–2,070 K; (3,591–1,797 °C) | Defined by water absorption,[chuckles]required] and commonly used in fiber optic telecommunications due to low attenuation losses in the SiO 2 glass medium (sylice). Image intensifiers are sensitive to this spectrum area; examples include night vision devices such as night vision glasses. Near infrared spectroscopy is another common application. |
| Shortwave Infrared | SWIR, IR-B DIN | 1.4–3 μm | 100-214 THz | 413–886 meV | 2.070–966 K (1.797–693 °C) | Water absorption significantly increases to 1450 nm. The range from 1530 to 1560 nm is the dominant spectral region for long-distance telecommunications. |
| Infrared of average wavelength | MWIR, IR-C DINMidIR. Also called intermediate infrared (IIR) | 3–8 μm | 37–100 THz | 155–413 meV | 966–362 K (693–89 °C) | In guided missile technology, the 3-5 μm part of this band is the atmospheric window in which the reference heads of passive IR missiles are designed to operate heat search heading for the infrared signature of the target plane, usually the reaction motor. I'm sorry to escape. This region is also known as thermal infrareds. |
| Long wavelength Infrared | LWIR, IR-C DIN | 8–15 μm | 20–37 THz | 83-155 meV | 362-193 K
(89 - −80 °C) | The "thermal images" region, in which the sensors can obtain a completely passive image of objects with a temperature only slightly higher than ambient temperature, for example, the human body, based solely on thermal emissions and which do not require lighting such as sun, moon, or infrared illuminator. This region is also called "thermal infrareds". |
| Infrared far away | FIR | 15–1,000 μm | 0.3–20 THz | 1.2–83 meV | 193-3 K
(−80.15 - −270.15 °C) | (see also distant infrared laser and distant infrared) |
NIR and SWIR are sometimes called "reflected infrared" while MWIR and LWIR are sometimes called "thermal infrared". Due to the nature of blackbody radiation curves, typical 'hot' objects, such as exhaust pipes, often appear brighter in the MW compared to the same object seen in the LW.
CIE Division Scheme
The International Commission on Illumination (CIE) recommended the division of infrared radiation into the following three bands:
| Abbreviation | Wave length | Frequency |
|---|---|---|
| IR-A | 700 nm - 1,400 nm (0.7 μm - 1.4 μm) | 215 THz - 430 THz |
| IR-B | 1,400 nm – 3,000 nm (1.4 μm - 3 μm) | 100 THz - 215 THz |
| IR-C | 3,000 nm - 1 mm (3 μm – 1,000 μm) | 300 GHz - 100 THz |
ISO 20473 Scheme
ISO 20473 specifies the following schema:
| Designation | Abbreviation | Wave length |
|---|---|---|
| Near infrared | NIR | 0.78–3 μm |
| Infrared medium | MIR | 3–50 μm |
| Infrared far away | FIR | 50–1000 μm |
Astronomy Division Outline
Astronomers often divide the infrared spectrum as follows:
| Designation | Abbreviation | Wave length |
|---|---|---|
| Near infrared | NIR | 0.7 to 2.5 μm |
| Infrared medium | MIR | 3 to 25 μm |
| Infrared far away | FIR | higher than 25 μm. |
These divisions are not precise and may vary by publication. The three regions are used for the observation of different temperature ranges[citation needed], and therefore of different environments in space.
The most common photometric system used in astronomy assigns capital letters to different spectral regions depending on the filters used; I, J, H, and K cover near-infrared wavelengths; L, M, N, and Q refer to the mid-infrared region. These letters are commonly understood in reference to atmospheric windows and appear, for example, in the titles of many scholarly articles.
Division scheme according to sensor response
A third scheme divides the band based on the response of various detectors:
- Near infrared: from 0.7 to 1.0 μm (from the approximate end of the response of the human eye to the silicon).
- Shortwave Infrared: From 1.0 to 3 μm (from silicon cut to the MWIR atmospheric window). InGaAs covers up to about 1.8 μm; less sensitive lead salts cover this region.
- Midwave Infrared: From 3 to 5 μm (defined by the atmospheric window and covered by Indian antimoniide [InSb] and cadmium and mercury (HgCdTe) and partly by lead seleniide [PbSe]).
- Longwave infrared: 8 to 12, or 7 to 14 μm (it is the atmospheric window covered by HgCdTe and microballometer).
- The very long wave infrared (VLWIR) (approximately 12-30 μm, covered by doped silicon).
Near infrared is the region closest in wavelength to radiation detectable by the human eye. Mid-infrared and far-infrared gradually move away from the visible spectrum. Other definitions obey different physical mechanisms (emission peaks, vs. bands, water absorption) and the most recent ones obey technical reasons (common silicon detectors are sensitive up to about 1,050 nm, while the sensitivity of InGaAs starts around 950 nm and ends between 1,700 and 2,600 nm, depending on the specific configuration). There are currently no international standards for these specifications.
The start of infrared is defined (according to different standards) at various values, typically between 700 and 800 nm, but the boundary between visible and infrared light is not precisely defined. The human eye is noticeably less sensitive to light above the 700nm wavelength, so longer wavelengths contribute negligibly to scenes illuminated by common light sources. However, particularly intense near-infrared light (for example, from IR lasers, IR LED sources, or bright daylight with visible light removed by color gels) can be detected down to about 780 nm, and will be perceived like red light. Intense light sources providing wavelengths up to 1,050 nm can be seen as a dull red glow, causing some difficulty in illuminating near-infrared scenes in the dark (this practical problem is usually solved with indirect illumination).). Leaves are particularly bright in the near IR, and if all visible light leaks around an IR filter are blocked, and the eye is given a moment to adjust to the extremely dim image coming through a visually opaque photographic filter passing through the IR, it is possible to see the Wood effect consisting of the foliage that glows in the IR.
Infrared telecommunication bands
In optical communications, the portion of the infrared spectrum that is used is divided into seven bands based on the availability of light sources that transmit/absorb materials (fibers) and detectors:
| Banda | Description | Wave length range |
|---|---|---|
| Band O | Original | 1,260–1,360 nm |
| Band E | Extended | 1,360–1,460 nm |
| Banda S | Short wavelength | 1,460-1,530 nm |
| Band C | Conventional | 1,530–1,565 nm |
| Banda L | Long wavelength | 1,565–1,625 nm |
| Banda U | Ultralong wavelength | 1,625–1,675 nm |
The "C-band" is the dominant band for long-distance telecommunications networks. The S and L bands are based on less established technology and are not as widespread.
Heat radiation
Infrared radiation is popularly known as "thermal radiation," but light and electromagnetic waves of any frequency heat up surfaces that absorb them. Infrared light from the Sun accounts for 49% of Earth's heating, with the remainder being caused by visible light being absorbed and then re-radiated at longer wavelengths. The visible or ultraviolet light emitted by the laser can char paper, and incandescent objects emit visible radiation. Objects at room temperature will emit spontaneously concentrated radiation mostly in the 8 to 25 μm band, but this is not unlike the emission of visible light by incandescent objects and ultraviolet by even hotter objects (see blackbody and displacement law). of Wien).
Heat is energy in transit that flows due to a difference in temperature. Unlike heat transmitted by thermal conduction or thermal convection, thermal radiation can propagate through a vacuum. Thermal radiation is characterized by a particular spectrum of many wavelengths that are associated with the emission of an object, due to the vibration of its molecules at a given temperature. Thermal radiation can be emitted by objects at any wavelength, and at very high temperatures such radiation is associated with spectra much higher than infrared, extending to the visible, ultraviolet, and even X-ray regions (for example, the corona solar). Therefore, the popular association of infrared radiation with thermal radiation is just a coincidence based on the typical (comparatively low) temperatures usually found near the surface of planet Earth.
The concept of emissivity is important to understand the infrared emissions of objects. This is a property of a surface that describes how its thermal emissions deviate from the idea of a blackbody. To further explain it, two objects at the same physical temperature may not display the same infrared image if they have different emissivities. For example, for any preset emissivity value, objects with a higher emissivity will appear hotter, and those with a lower emissivity will appear cooler (assuming, as is often the case, that the surrounding environment is cooler than the objects). objects being viewed). When an object does not have perfect emissivity, it acquires reflectivity and/or transparency properties, so that the surrounding temperature is partially reflected and/or transmitted through the object. If the object were in a hotter environment, then a lower emissivity object at the same temperature would probably appear hotter than a more emissive one. For this reason, incorrect selection of emissivity and disregard of ambient temperatures will give inaccurate results when using infrared cameras and pyrometers.
Uses of infrared rays
Infrared is used in night vision equipment when the amount of visible light is insufficient to see objects. The radiation is received and then reflected on a screen. The hottest objects become the most luminous.
A very common use is made by remote controls (or telecommands) that generally use infrared instead of radio waves since they do not interfere with other signals such as television signals. Infrared is also used to communicate over a short distance between computers and their peripherals. Devices that use this type of communication generally comply with a standard published by the Infrared Data Association.
The light used in optical fibers is generally infrared.
Near Infrared
The near infrared is the shortest wavelength region of the infrared spectrum, located between visible light and mid-infrared, approximately between 800 and 2,500 nanometers, although there is no universally accepted definition.
Astronomy
In astronomy, near-infrared spectroscopy is used to study the atmospheres of cool stars. In this range, rotational and vibrational transition lines of molecules such as titanium oxide, cyanogen and carbon monoxide can be observed, which give information about the spectral type of the star. It is also used to study molecules in other astronomical objects, such as molecular clouds.
Industrial infrared emitters
Another of the many applications of infrared radiation is the use of infrared emitting equipment in the industrial sector. In this sector the applications occupy an extensive list but its use can be highlighted in applications such as drying of paints or varnishes, drying of paper, thermosetting of plastics, preheating of welds, curvature, tempering and lamination of glass, among others. The irradiation on the material in question can be prolonged or momentary, taking into account aspects such as the distance from the emitters to the material, the speed of passage of the material (in the case of production lines) and the temperature to be achieved.
Generally, when talking about infrared emitting equipment, four types are distinguished depending on the wavelength they use:
- Short-wave infrared transmitters.
- Quick-wave infrared transmitters.
- Mid-wave infrared transmitters.
- Long-wave infrared transmitters.
Night vision
Infrared is used in night vision equipment when there is not enough visible light to see. Night vision devices work through a process that involves converting photons in ambient light into electrons that are then are amplified by a chemical and electrical process and converted back to visible light. Infrared light sources can be used to increase the ambient light available for conversion by night vision devices, increasing visibility in the dark without using actually a visible light source.
The use of infrared light and night vision devices should not be confused with thermal imaging, which creates images based on surface temperature differences by detecting infrared radiation (heat) emanating from objects and their surroundings..
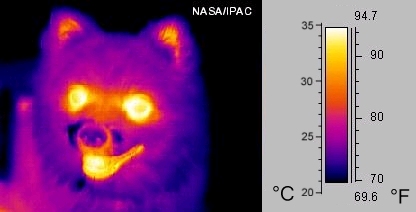
Thermography
Infrared radiation can be used to determine the temperature of objects from a distance (if the emissivity is known). This is called thermography, or in the case of very hot objects in the NIR or visible it is called pyrometry. Thermography (thermal imaging) is used primarily in military and industrial applications, but the technology is finding its way onto the public market in the form of in-car infrared cameras due to greatly reduced production costs.
Thermal imaging cameras detect radiation in the infrared range of the electromagnetic spectrum (approximately 9,000 to 14,000 nanometers or 9-14 μm) and produce images of that radiation. Since infrared radiation is emitted by all objects as a function of their temperature, according to the law of black body radiation, thermography makes it possible to "see" the environment with or without visible lighting. The amount of radiation emitted by an object increases with temperature, so thermography makes it possible to see variations in temperature (hence its name).
Hyperspectral Imaging
A hyperspectral image is an "image" containing a continuous spectrum across a wide spectral range in each pixel. Hyperspectral imaging is gaining importance in the field of applied spectroscopy, especially in the NIR, SWIR, MWIR, and LWIR spectral regions. Typical applications include biological, mineralogical, defense, and industrial measurements.
Hyperspectral imaging in the thermal infrared can be done in a similar way using a thermal imaging camera, with the key difference that each pixel contains a full LWIR spectrum. Consequently, the chemical identification of the object can be carried out without the need for an external light source, such as the Sun or the Moon. These types of cameras are often applied for geological measurements, outdoor surveillance and UAV applications.
Other images
In Infrared Photography, infrared filters are used to capture the near-infrared spectrum. Digital cameras often use infrared blockers. Cheaper digital cameras and camera phones have less effective filters and can "see" the intense near-infrared, appearing as a bright purple-white color. This is especially pronounced when taking photos of subjects near areas of infrared light (such as near a lamp), where the resulting infrared interference can wash out the image. There is also a technique called 'T-Rays', which consists of obtaining images using far-infrared or terahertz radiation. The lack of bright sources can make terahertz photography more difficult than most other infrared imaging techniques. In recent times, T-ray imaging has aroused great interest due to a number of new developments, such as “time-domain terahertz spectroscopy”.
Follow-up
Infrared tracking, also known as infrared homing, refers to a Passive Missile Guidance System, which uses a target's emission of electromagnetic radiation in the infrared part of the spectrum to track it. Missiles that use infrared seeking are often referred to as "heat seekers" since infrared (IR) is just below the visible spectrum of light in frequency and is strongly radiated by hot bodies. Many objects, such as people, vehicle and aircraft engines, generate and retain heat, and as such are especially visible in the infrared wavelengths of light compared to objects in the background.
History of Infrared Science
The discovery of infrared radiation is attributed to William Herschel, the astronomer, in the early 19th century century. Herschel published his results in 1800 before the Royal Society of London. Herschel used a prism to refract sunlight and detected the infrared, beyond the red part of the spectrum, through an increase in temperature recorded on a thermometer. He was surprised at the result and called them "heat rays."The term "infrared" it did not appear until the late 19th century century.
Other important dates are:
- 1737: Émilie du Châtelet predicted what is now known as infrared radiation in Dissertation sur la nature et la propagation du feu.
- 1830: Leopoldo Nobili made the first thermomile Detector infrared.
- 1840: John Herschel produces the first thermal image, called thermogram.
- 1860: Gustav Kirchhoff formulated theorem of the black body E=J(T,n){displaystyle E=J(T,n)}.
- 1873: Willoughby Smith discovered the selenium photoconductivity.
- 1878: Samuel Pierpont Langley invents the first bolometer, a device capable of measuring small temperature fluctuations and, therefore, the power of the sources of the distant infrared.
- 1879: The Stefan-Boltzmann Act empirically formulated that the power radiated by a black body is proportional to T 4.
- Decade of 1880 and 1890: Lord Rayleigh and Wilhelm Wien resolved part of the equation of the black body, but both solutions diverged in parts of the electromagnetic spectrum. This problem was called "castrophe of the ultraviolet and infrared catastrophe."
- 1892: Willem Henri Julius published the infrared spectra of 20 organic compounds measured with a bolometer in angular displacement units.
- 1901: Max Planck published the equation of the black body and theorem. It solved the problem by quantifying the allowed energy transitions.
- 1905: Albert Einstein developed the theory of the photoelectric effect.
- 1905-1908: William Coblentz published infrared spectra in wavelength units (micrometers) for several chemical compounds in Investigations of Infra-Red Spectra.
- 1917: Theodore Case developed the drill sulfide detector; a British scientist built the first infrared search engine and tracker (IRST) capable of detecting planes at a distance of one mile (1.6 km).
- 1935: Lead sales: first missile guide in World War II.
- 1938: Yeou Ta predicted that the pyroelectric effect could be used to detect infrared radiation.
- 1945: Zielgerät 1229 "Vampir" was introduced as the first portable infrared device for military applications.
- 1952: Heinrich Welker cultivated InSb synthetic crystals.
- Decades of 1950 and 1960: Nomenclature and radiometric units defined by Fred Nicodemenus, G. J. Zissis and R. Clark; Robert Clark Jones defined the D.
- 1958: W. D. Lawson (Royal Radar Establishment in Malvern) discovered the infrared detection properties of cadmium and mercury (HgCdTe).
- 1958: the Falcon and Sidewinder missiles were developed with infrared technology.
- 1960s: Paul Kruse and his colleagues at the Honeywell Research Center demonstrate the use of HgCdTe as an effective compound for infrared detection.
- 1962: J. Cooper demonstrated pyroelectric detection.
- 1964: W. G. Evans discovered infrared thermoreceptors in a pyramid beetle.
- 1965: First infrared manual; first commercial imaging generators (Barnes, Agema (now part of FLIR Systems Inc.); the reference text of Richard Hudson; F4 TRAM FLIR of Hughes; pioneer phenomenology of Fred Simmons and A. T. Stair; formed the American army's night vision laboratory (now Night Vision and Electronic Sensors Directorate (NVESD) and Rache detection
- 1970: Willard Boyle and George E. Smith propose the CCD at Bell Laboratories for the videochat.
- 1973: Common module program initiated by the NVESD.
- 1978: Infrared image astronomy reaches the age of majority, observatories are planned, NASA's infrared telescope is opened in Mauna Kea; sets of 32 × 32 and 64 × 64 are produced using InSb, HgCdTe and other materials.
- 2013: On 14 February, researchers developed a brain implant that gives rats the ability to perceive infrared light, which for the first time provides living beings with new skills, rather than simply replacing or increasing existing skills.
Contenido relacionado
Decisecond
Henri becquerel
Levi-Civita Connection