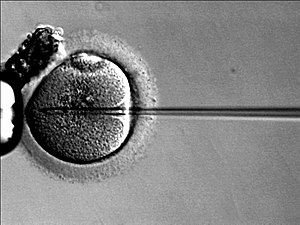
In vitro fertilization
In vitro fertilization, IVF or (IVF in English) is a technique by which the fertilization of oocytes by spermatozoa It takes place outside the mother's body. IVF is the main treatment for infertility when other assisted reproductive methods have not been successful. The process involves hormonal control of the ovulatory process, extracting one or several oocytes from the maternal ovaries, to allow them to be fertilized by sperm in a liquid medium. The fertilized egg (zygote) can then be transferred to the woman's uterus, where it implants in the uterus and continues to develop until delivery.
In vitro
The term in vitro is a Latin term meaning 'in glass'. It is used because in the first biological experiments in which tissue cultures were carried out outside the living organisms from which they came, they were carried out in glass containers, such as test tubes, test tubes or Petri dishes. Today, the term in vitro refers to any biological procedure that is performed outside the organism in which it would normally take place, to distinguish it from an in vivo experiment where the Tissue remains within the living organism in which it is normally found. Babies conceived through IVF were colloquially referred to as test tube babies, referring to glass or plastic containers called test tubes, which are frequently used in chemistry and biology laboratories. However, normally in vitro fertilization is performed in flat dishes called Petri dishes; the most often used petri dishes are made of plastic, however the name IVF still sticks.
Indications
Initially IVF was developed to overcome infertility situations due to problems in the fallopian tubes, but later it was observed that the technique was also successful in other cases of infertility. The introduction of intracytoplasmic sperm injection (ICSI) largely solves the problems of male infertility.
For IVF treatment to be successful, it is necessary to have healthy oocytes, sperm that can fertilize them, and a uterus that can sustain a pregnancy. Although in some countries IVF treatments are covered by social health services, this technique is usually used when other options have failed, because IVF carries high costs.
IVF can also be used in menopausal women, using oocytes from a donor. It is also a technique that can be considered in patients who have suffered a total or partial loss of fertility due to aggressive treatment for a serious pathology (such as cancer).
Method
Ovarian stimulation
Prior to in vitro fertilization, the development of multiple follicles in the ovaries is generally stimulated on the third day of menstruation by hormonal treatments. Gonadotropin injections (usually FSH analogues) are used in most patients, and some complementary analysis of hormonal levels such as estradiol or progesterone, and follicular growth can be performed using gynecological ultrasonography. The necessary stimulation time is variable, usually 8-12 days of injections are needed. Spontaneous ovulation during the cycle is prevented by the use of GnRH agonists (aGnRH) or GnRH antagonists (agGnRH), which block the spontaneous surge of luteinizing hormone (LH). Both generate, in other words, what is known as reversible hypogonadotrophic hypogonadism. However, GnRH agonists differ from antagonists mainly because their effect is not immediate, but instead triggers a surge of FSH and LH in the first instance (flare-up effect), producing a subsequent block in the release of gonadotropins by GnRH receptor saturation of the activation cascade. However, there are different stimulation protocols that vary on the start day, medications used, and methods to prevent and induce the luteinizing hormone (LH) surge. Currently, a recombinantly generated FSH analogue, corifollitropin alfa, is also being used as an ovulation stimulant. This molecule contains a fragment of human chorionic gonadotropin, which gives it a very favorable pharmacokinetic profile, lowering drug doses compared to conventional FSH.
Basically, if in the laboratory the team opts for a treatment with aGnRH, they can choose between a short and a long protocol:
- Long protocol: GnRH agonists are given to the patient several days before the new cycle and during the administration of exogenous gonadotropins. Among its multiple advantages, it stands out that, with it, the premature release of LH is not ensured, which guarantees the invalidation of all early ovulation. In turn, this fact allows the embryologist to plan the date of follicular uptake without margin of error. On the other hand, it ensures that the development of the follicles is synchronized. Unfortunately, with the application of this protocol, the probability of ovarian hyperstimulation syndrome (OHSS) is multiplied, and luteal phase support (progesterone administration) is required, as well as a greater amount of gonadotropins.
- Short protocol: GnRH agonists begin to be administered in the first days of the cycle, almost at the same time as exogenous gonadotropins. The main virtue of this procedure is that it achieves better results in women with a low response, although this fact has not yet been fully demonstrated. On the other hand, the application of this protocol multiplies the risk of LH surges (thus inducing early ovulation) and does not allow synchronous development of the follicles. In short, much less control over follicular development is established.
On the other hand, when GnRH antagonists are used, only one protocol is established, in which the blocking substance is administered several days after the start of the cycle, at the same time as recombinant or purified gonadotropins are supplied from urine.
Ovarian stimulation protocols have become complex and expensive. For this reason, there seems to be a worldwide trend aimed at reducing the amount and dose of drugs used during stimulation in order to reduce the risks and costs associated with these treatments [6]. Examples of these efforts are the IVF treatments with Natural Cycles, in addition to the IVF protocols with minimal stimulation developed by the New Hope Fertility groups and the Kato Ladies Clinic, directed by doctors John Zhang in NY [7], Dr. O Kato in Tokyo [8], and Dr. Chávez-Badiola in Mexico [9].
Oocyte retrieval
When follicle maturation is considered adequate, the patient is given human chorionic gonadotropin (β-hCG) or a GnRH agonist. The first acts as an analogue of luteinizing hormone (LH); while the second induces a shot of the luteinizing hormone (LH) itself. In either case, the drug will cause ovulation around 36 hours after injection, but the withdrawal procedure takes place just before this occurs.
Oocyte retrieval is scheduled about 36 hours after ovulation induction and is performed transvaginally, using an ultrasound-guided needle, which punctures the vaginal wall to reach the ovaries. A doctor aspirates the follicles with the help of an ultrasound scanner and collects the follicular fluid in tubes that will be introduced into a thermoblock until they go to the laboratory. Follicular fluid is a yellowish, serous fluid that contains lymphocytes and granulosa cells singly or in clusters with or without oocytes. As the ovary is punctured, the follicular fluid turns red (bloody) due to bleeding from the puncture. The blood is toxic to the oocyte as it contains many antibodies, so once the puncture is finished it will have to be removed. This step is carried out in the laboratory, where the puncture fluid is processed in order to recover the oocytes contained in the fluid; in this way the oocytes will be obtained, they will be washed and they will be classified according to their morphology. These three steps must be carried out in the shortest possible time to avoid the effect of temperature, to which oocytes are very sensitive, and the damage caused by the blood fluid.
- Temperature: The egg is the most sensitive element to the temperature of the entire laboratory. It has to be at 37 °C in the incubator, so a single degree of difference is enough for the egg to be denaturalized. In fact, if it's under 34 °C the meitic spine will depollute, and when it becomes shaped it can create chromosomal abnormalities, so that the egg will be fruitful but will not lead to a normal embryo.
Cumulus-crown-oocyte complexes that can be seen with the naked eye (several mm in diameter) must be isolated.
Thus, once the oocytes have been extracted, the sample is looked at under an optical microscope to select the oophoric clusters. Then, these are cleaned of the granulosa cells to be left with only the oocyte. This process is known as denudation.
- Media: during this process different means of cultivation are used with different composition:
- In the tubes: 0.1 ml of tamponated medium (HEPES) with heparin to avoid the formation of clots.
- The HEPES medium (which should be from the day before being used in a stove at 37 °C) will temporarily accumulate the clusters while the puncture is done. It will also be used to wash and reduce the size of the clusters before passing to the incubator.
- Crop plates: with a simple medium rich in glucose (e.g. HTF + HSA 10 mg/ml) to keep them in the incubator at 5% carbon dioxide. The medium should be from the day before being used in the incubator at 37 °C and 5 % of carbon dioxide.
The punctures are normally scheduled every 30 minutes, although the search for the oocytes does not usually last more than 15 minutes. In these processes, local, general or partial anesthesia is used to avoid the pain produced by the puncture.
There are 3 stages of maturation in the clusters that are extracted by follicular puncture, namely:
- Grade I (MII nuclear maturation): The oocyte cumulus and crown present an expanded appearance. It is the state in which the oocyte presents a greater maturation and only those of this type are used in assisted reproduction techniques.
- Grade III (nuclear maturation VG): The oocyte stands out for the great compaction of the cumulus, which has few cells, very attached to the zona pellucida (ZP).
- Grade II (MI nuclear maturation): The oocyte presents an intermediate appearance between the two previous stages.
This classification tries to describe the oocyte and its state of nuclear maturation being surrounded by cumulus and crown cells.
- Morphology: to observe well the ovocyte would have to place it in very little amount of medium to spread the granulous cells. So the advantage of knowing the mature state does not take any benefit from the manipulation of the power to know it.
That is why this classification is currently not very relevant and should not be given greater importance, except to indicate characteristics that are especially out of what is considered normal: clusters or crowns with an apoptotic or postmature appearance, presence of blood, etc.
Fecundation
Once in the laboratory, the extracted cumulus crown oocyte complexes are washed in HEPES medium to maintain pH, trimming the surrounding granulosa cells and preparing them for fertilization. The oocytes must remain in the incubator for at least 4 hours (simple medium rich in glucose) until they are inseminated, that is, approximately 40 hours after ovulation induction, which would be the moment of spontaneous ovulation. This time is necessary to have an appropriate maturation of the oocyte and to simulate the natural conditions that occur in the uterus. If the insemination is performed before or after this period of time, the insemination efficiency will decrease.
At the same time, the semen is prepared for fertilization, eliminating the inactive cells, the seminal fluid and its capacitation is carried out. The adequate parameters of qualified semen for IVF are: 8-10 million spermatozoa per milliliter, more than 75% of progressive motile spermatozoa and more than 1% of normal forms. If the semen comes from a donor, it will probably have been prepared before it was frozen and quarantined, and when it is thawed it will be ready to use. Higher sperm concentrations can cause abnormal fertilizations (polyspermy) and a lower sperm concentration can cause fertilization failures.
There are different IVF protocols but they are all based on the same principle: sperm and oocyte are incubated together (in a ratio of approximately 75,000:1) in a simple culture medium with glucose for about 18 hours. The oocyte will be fertilized during the first 20 minutes of exposure. To fertilize an oocyte, a single sperm is not enough, several dozen are necessary to break down the zona pellucida (protective cell layer). In this sense, the first sperm to arrive is not usually responsible for fertilization.
If the semen sample has lower values than the above, ICSI is used instead of IVF. ICSI is also known as microinjection. It consists of directly injecting the sperm into the oocyte. It is the most efficient technique when the spermatozoa are severely damaged, that is, when there is a serious problem of infertility in men.
However, due to the high success rate - there is a higher fertilization rate with this technique compared to conventional IVF - ICSI is the most widely used worldwide. While with ICSI 8/1000 oocytes are fertilized, with conventional IVF only 4-6/1000 are fertilized. Therefore IVF is theoretically the first option to be tried due to its physiological similarity but ICSI is generally preferred for its efficiency.
After 16-18 hours, fertilization is verified, which should have already occurred. Some authors wash the oocytes after half an hour to avoid exposure to ROS, which can be formed by the presence of dead sperm.
From 15 to 20 hours after conception, the human zygote remains in the pronucleus (PN) stage. Fertilization is considered to be correct when the zygote has two NP and two corpuscles (CP). Sometimes it is difficult to interpret the CP and any embryo with two NPs is assessed as correctly fertilized. Any other combination is considered abnormal and is discarded. Most of the time the paternal NP appears first in the central position and the maternal one comes later. In order to carry out fertilization correctly, it is necessary to previously decumulate or denude the zygote. This process consists of removing the cells of the Cumulus Oophorus, or cluster of the ovule. This technique makes it possible to know the exact stage of maturation of the oocytes, which is essential to perform intracytoplasmic sperm injection (ICSI) in In Vitro Fertilization (IVF). Denudation consists of two phases: a first enzymatic phase, where hyaluronidase is used to break the cumulus cell junctions; and another mechanical, consisting of the detachment of the cluster by passing through Pasteur pipettes (or other tools for the same purpose) of different calibers.
The fertilized egg is transferred to a simple culture medium (such as HTF/HSA) or sequential (G1 from Vitrolife) and is kept for around 48h until it reaches the 6-8 cell stage.
Studies have been carried out that show that freeze-drying mouse sperm allows the development of normal embryos after the injection of oocytes.
Embryo culture
Once the egg has been fertilized and a zygote has been obtained, it is cultivated to promote cell division and growth to give rise to an embryo. This culture lasts between 2 and 5 days, and it is very important that it is carried out in optimal conditions for the embryo, since its quality and its implantation rate when it is transferred to a uterus will depend on this.
Growing media
In order for the embryo to grow in the best possible conditions, different types of culture medium are used:
Simple media: simple in composition and easy to prepare. The composition of these media is determined from the theoretical composition of the tubal fluid (HTF or P1 media) or from the composition of culture media for mouse zygote development (KSOM, Earle, M16, and T6 media). and are usually supplemented with maternal serum or human serum albumin (HSA). They are optimal for the initial growth of the embryo (up to 3 days of culture). From day four they do not guarantee optimal development due to the beginning of the active transcription of the embryo, for which substances that these media do not contain are required. Embryos are usually cultured for 3 days prior to implantation, after which they reach a 6-8 cell stage. This allows the embryologist to monitor its cell division rate and gene activation to ensure that the embryo is viable and will implant properly. The time of implantation will only be brought forward, normally after two days of culture, when the couple undergoing IVF have few embryos available for transfer or when the embryos develop slowly.
Complex media: its composition is more complex, it includes vitamins, amino acids, metals, serum,... These are commercial media that have been designed for the cultivation of somatic cells in culture, such as the medium Ham F10 media, so while they improve embryonic development to blastocyst (day 5) compared to plain media, they are not optimized for embryo culture. After five days of culture, the embryo reaches the blastocyst stage, in which it is made up of 12-16 cells and has a high implantation rate. They are usually cultured up to this stage when miscarriages or implantation failures have previously occurred in the patient.
Sequential means: they take into account the fact that the embryo goes through different environments from the time it is fertilized in the fallopian tube until it reaches the uterus. The sequential media are composed of three types of media: one medium for gamete preparation (plain medium), another for development up to day 3 (G1 medium), and a third to reach the blastocyst stage (G2 medium). These media are optimized for the development of embryos up to the blastocyst, but they are more sensitive to temperature, and therefore more unstable.
The plates with which they are worked are first bathed in a pre-washed paraffin oil that is used as a protective barrier to prevent rapid evaporation of the medium, to keep the samples safe from external contaminants during in vitro culture and to help to maintain uniform temperature in the medium. In this, drops are added with the hydrophilic medium that will be in which the oocytes are kept, never in contact with the oil.
Incubators
The incubators provide the appropriate culture medium and environment, controlling the conditions of temperature, light and pH. This will be essential for correct embryonic development to take place and, therefore, for obtaining good quality embryos. The type of incubator used will determine the embryo selection. During development it will be essential to analyze the morphological characteristics of the embryo in different stages, specifically in the first 6 days. Some parameters that embryologists evaluate are the number of cells, the rate of division, the size and symmetry of the cells, the percentage of fragmentation...
There are basically two types of incubators, conventional and time-lapse.
- Conventional incubators: these devices reproduce the optimal conditions for embryo development. However, to make the morphological evaluation it is necessary to take the embryos out of the incubator daily to be able to observe them under the microscope, exposing them to temperature variations and other harmful agents that can reduce the quality of the embryo.
- Incubators time-lapse: the time-lapse systems such as Geri Plus or Embryoscope are incubators that, in addition to reproducing the appropriate conditions for cultivation, have a camera. This will allow us to observe embryonic development without the need to remove embryos daily to analyze them under a microscope. In addition to avoiding exposure to radical changes in the conditions of cultivation, these devices allow us to obtain information of all the embryonic development minute to minute and not only at the time of observation, as in the previous case. This will allow a much more comprehensive selection of embryos to be transferred.
Morphological changes in the embryo
In some cases in assisted reproduction, it is decided to keep the embryo in culture until day 5 or 6 instead of transferring it on day 3. Long culture has some advantages such as better selection of the embryo or increasing the rate of implantation. Although there are also some drawbacks such as the high risk of embryo blockage. Thus, a series of continuous changes are observed in the embryo that are classified into stages:
- Mórula (M): embryo of more than 12 cells without compacting at all. Present in Day 4.
- Compact formula (MC): day 4 or 5. It is an embryo with 16 cells or more, but in which the cells are no longer differentiated, the blastocele or throphoectoderm cells are not differentiated.
- Early Blast: embryo on day 4 or 5 in which the flat cells of the trophoectoderm are distinguished on the surface of the embryo and a cavity less than 50% of the volume of the embryo.
- Blastocisto cavitado: is the typical stage of day 5. The trophoectoderm and a blastocele are clearly distinguished that occupies at least half of the inside of the embryo.
The diameter of the embryo remains around 140 microns. Not always distinguishing the internal cell mass.
- Expanded Blastocisto: Appreciated in Day 5 or 6. Trophoectoderm, blastocele and internal cell mass are distinguished. The diameter is greater than 150 microns and the pelvic area is tuned.
- Blastocisto initiating eclosion (BHi): It is an expanded blastocyst in which a hernia is distinguished from where the eclosion is beginning.
- Blastocisto eclosionado (BH): it is a blastocyst entirely outside the pelvic zone, with a diameter normally greater than 300 microns, more than double than in the previous stage. It's the slowest stadium you can keep in vitro.
The morphology of the inner cell mass and trophectoderm is used as embryo quality criteria.
To carry out the long culture of embryos, sequential media or cocultivation on a monolayer of endometrial cells are used.
It is the latest stage of the embryo that can be maintained in vitro.
IVF Laboratory
There is no consensus on what a laboratory for in vitro fertilization should be like. It would be appropriate for it to be a clean room with full control of all parameters and with the fewest possible number of horizontal surfaces, but this is currently not the case due to its high cost. Even so, it is necessary to control certain parameters, because although the embryos are strong and robust, their implantation rate is influenced by environmental conditions. The most common parameters controlled in an IVF laboratory are:
- -Temperatura: incubators must be at 37 °C, so you must always keep them on and prevent external conditions from varying, as the incubator will tend to balance with these; for this it will be necessary to keep on the thermostat of the laboratory (external conditions) 24 hours with a stable temperature of about 21-24 °C (consensus with the staff to see how they are more comfortable)
- -pH: It is very important to incubate gametes and cultivate embryos in culture media with a pH similar to their internal pH (pH=7, 2). When the difference between these is very different the development rate decreases. Most of the means of cultivation are prepared using bicarbonate as pH buffer, controlling it by balance between atmospheric CO2, dissolved CO2, bicarbonate and hydrogen ions in solution. The main purpose of maintaining CO2 levels in the incubator is to maintain the pH of the medium. Some authors believe that the pH of the medium can be measured as an indirect measure of CO2 levels.
It can be done with a blood gas analyzer or a calibrated pH meter just before measuring an aliquot kept inside the incubator. The aliquot of medium used to measure the pH should be discarded.
- -Particles: there is communication between the O.R. and the lab so we must avoid this possible contamination. To do this, we have:
- Sterility and asepsia.
- Filters for air conditioning. The best are those that have to be replaced every time, such as HEPAs in laminar flow booths, those that have a duration of 4 months. In this way, we ensure that they are always clean and we do not expect to change them until they are saturated with dirt.
- Positive pressure, that is, the air direction is from inside out. This way, it drags out everything that comes in and prevents you from entering the outside.
-VOCs, such as hydrogen, oxygen, fluoride, bromine or nitrogen. VOC sources are oils, solvents, cleaning products and other compounds such as benzene, formaldehyde, toluene... Special care must be taken not to wear deodorants (only roll-on) or perfumes are allowed.
The location of the center is very important. The surroundings should be as uncontaminated as possible. For example, a gas station near the center is dangerous. Volatile organic compounds (VOCs) should be minimized, as they could have embryotoxic (embryo-damaging) effects. Some VOCs are not filterable by normal methods, and therefore activated carbon filters with different concentrations of potassium permanganate must be used at the entrance of any gas in the laboratory. It is necessary to emphasize that these filters have a limited life due to their absorption capacity, so they must be changed periodically.
There are also other parameters that can be controlled, although they are not as important as the previous ones:
- -Light: it works with normal light not very intense, and it must be avoided that it directly affects the plate with the embryo. To get this last, you have lomas that change the direction of light. On the other hand, comment that with embryos of mice it does work with darker lights because otherwise it would affect the division. However, in humans it is not shown that low-intensity lights diminish the viability of the embryo, so work with normal intensity lights and thus avoid vision problems derived from working in penumbra.
- - Relative Humidity (HR): does not directly influence work, but it does affect the growth of fungi. As their control is very expensive, only the laboratories that really need it (HR/200590%) have air conditioners for it. For comfort, HR should be around 50%.
In these types of laboratories, people always wear a mask. The use of surgical caps is also essential. These caps must cover all the hair, from the forehead to the bottom, so that the samples and the environment are not contaminated.
When it comes to design and layout, these two parameters greatly influence the success rates of the laboratory. The materials used for the floor, walls and ceiling must always be noble (stainless steel, linoleum, ceramic, etc.), and the presence of horizontal surfaces must be avoided (so that dirt does not accumulate). Likewise, efforts should be made to have an entrance gate separated from the rest of the rooms: the main room (with the surgical, incubator and micromanipulation area distributed), the cryopreservation laboratory, the preparation laboratory, the andrology laboratory and the DPI laboratory.. The equipment of a laboratory for reproduction must consist of a laminar flow cabinet, an inverted microscope with 400 magnification and modular phase contrast on an anti-vibration table and a temporary incubator.
The work unit consists of:
- A laminar flow cabinet with a heated surface and 5% CO2. - 400x inverted microscope with micromanipulation on an anti-vibration table. - A temporary incubator in which the samples with which we are working are stored. After being treated, they return to the main hatchery.
Similarly, human resources are important to avoid as many mistakes as possible. There must be specialized personnel viewing the work of others. Those who work in an IVF laboratory include embryologists, laboratory technicians, and administrative staff.
Selection
Specialized IVF laboratories have developed scoring methods to judge the quality of oocytes and embryos. Typically, experts examine the symmetry of the embryo, the structural integrity of its cells, and overall growth two to five days after fertilization. Now scientists are beginning to analyze not only the embryo, but also the medium in which it grows. Some centers are using chemical analysis and mathematical formulas to create a "metabolic footprint" of a healthy embryo, which could be used as a barometer to estimate the survival potential of an embryo. Others are trying to analyze the proteins secreted by the embryos and to measure the amount of oxygen consumed, which is a common sign of growth.
Usually, embryos that have reached the 6-8 cell stage are transferred 3 days after collection. Sometimes, however, the embryos are kept in culture for a longer period (about 6 days), and the transfer is performed at the blastocyst stage, especially if many good-quality 3-day-old embryos are observed. But never exceeding 14 days after fertilization. Blastocyst stage transfers show better pregnancy rates.
The Association for the Study of Reproductive Biology (ASEBIR) specified in 2007 a classification with which to evaluate embryos before their transfer, based on the results obtained from studies carried out in national reproduction centers and published scientific literature. Based on them, the four categories established are:
- Category A: optimal. They are those who have a correct development and no feature of poor prognosis, so they will always be transferred or cryopreserved. In patients with good prognosis (women under 36 years of age and no adverse pathology or oocyte receptors) type A embryos tend to have 40-60% of implant possibilities.
- Category B: Good. They are good quality embryos with high implantability. The usual is that they have between 20 and 40% probability of implantation.
- Category C: Suboptims. They are the embryos that present a series of features that although they are associated with less viability are not completely discardable and will be transferred if there is no other with better morphology, so they are also known as accompanying embryos. They usually have less than half of the possibilities of implantation (1-20%) although it will always depend on the type and extent of the morphological anomalies they present.
- Category D: Not viable. They have an implantation capacity of 1% or less, even 0.1%. They include both in vitro evolutionary embryos, whose characteristics are related to a lack of implantation potential, and those that are blocked. They are not transferred practically in any case.
Assessment of embryonic quality
The objective of in vitro fertilization is to transfer the embryo with the best potential to achieve a pregnancy, and that must be selected from among all those available in an IVF cycle. There are different systems for the classification of embryos according to the stage of development in which they are evaluated, as well as the different associations or individuals that propose them. However, the conventional evaluation has shown to be subjective, not replicable, and little related to prognosis. As a consequence of the above, some groups are actively working on developing embryo selection and ranking systems, supported by sophisticated computer systems, and with the capacity to apply Artificial Intelligence, artificial vision, and deep learning (Deep Machine Learning) during the process.
An example of computer technologies used today in some laboratories is the system called ERICA (Embryo Ranking Intelligent Classification Assistant) [10]. This system uses artificial vision filters and Deep Learning to classify embryos at the blastocyst stage with the purpose of assisting embryologists in the selection of embryos. The studies so far published with these technologies suggest results promising.
On the other hand, the conventional selection and classification of embryos is based on the Morphological Assessment Criteria for Human Oocytes, Early Embryos and Blasts collected in the ASEBIR consensus. This consensus has been established by studying the morphological characteristics of the embryos and determining their implantation capacity. Thus, the morphological parameters that help determine embryo quality are the following: cell number, division rate, fragmentation, size and symmetry of blastomeres, multinucleation, cytoplasmic appearance, and zona pellucida. Studying all these parameters we can determine the quality of the embryo.
- Number of cells: The number of cells is one of the most important parameters. The number of optimal cells in day 2 is four cells and in day 3 is seven or eight cells. Generally, embryos within these limits are classified as type A embryos. The embryos that present between six and ten cells can be viable and are type B embryos. Embryons with five cells or more than 10 are considered type C embryos. However, embryos that present a high number of cells in day 2 and day 3 may have a high probability of presenting chromosomal alterations such as multinucleated aneuploids or blastomeras. The potential for implanting these embryos is very low so they are considered D-type embryos. On the other hand, embryos with a low number of cells in day 2 and day 3 are considered blocked and their implantation capacity is very low (type D). The following table shows the quality of the embryo depending on the number of cells in day 2 and 3:
| Quality group | Number of cells in day 2 | Number of cells in day 3 |
|---|---|---|
| A | 4 | 7 - 8 |
| B | 2 - 5 | 7 or more |
| B | 4 | 9 or more |
| C | 2 - 5 | 7 or more |
| C | 2 - 4 | 6 |
| C | 6 | 8 or more |
| C | 3 | 6 or more |
| D | 7 or more | Any value |
| D | Any number | 5 or less |
- Speed of division: The speed of division is another of the most important parameters. The normal rate of division is observed in an embryo that folds its cell number every 24 hours and is a feasibility prognosis. A higher rate is associated with chromosomal abnormalities, so the quality of the embryo is minimal. On the other hand a lower speed is related to the embryo blockage. An embryo is considered blocked when it does not increase its number of cells after a period of 24 h. This type of embryos also presents a very low implantation rate because it is considered non-viable embryo. The chromosomal block could be caused by chromosomal abnormalities of the embryo or by unappropriate laboratory conditions.
- Fragmentation: The third parameter in importance is fragmentation. The fragmentation consists of the generation of cytoplasm portions surrounded by membrane without nucleus. The causes of the occurrence of fragments are not known, it is estimated that it may be a consequence of the conditions of the crop environment or an inherent property of embryonic development. The size of the fragment, number and location are relatively important elements. The size of the fragment is estimated as the total volume of the embryo occupied by such fragments. Relatively small sizes are not associated with a deterioration in implantation capacity, while as the size increases the quality of the embryo decreases. It is considered a poor prognosis if the fragments occupy more than 1/3 of the volume of the embryo. The loss of embryo quality is based on the fact that these fragments prevent intercellular communication between blastomers, which is vital for correct development. We can sort the fragmentation into several types:
| Grade of fragmentation | Features |
|---|---|
| I | Fragmentation less than 5%.
Only an affected blastomera |
| II | Fragments partially distributed around the embryo, close to the periviteline space.
Total or partial fragmentation of a single blastomera |
| III | Small fragments, distributed around the embryo.
Several blastomeras from the periphery. |
| IV | Large fragments, distributed around the embryo.
Associated with irregular blastomeras. |
| V | Necrotic fragments. Associates to blastomeras with cytoplasm contracted |
Embryos with fragmentation of types I, II or III with a percentage of fragmentation less than 10% are considered type A (optimal); between 11-25% are considered type B embryos and between 26-35% are type C embryos (suboptimal); when the percentage of fragmentation is greater than 35% we are faced with type D (abnormal) embryos. If the fragmentation is type IV or V, the embryos are directly considered abnormal, regardless of the percentage of fragmentation. The number of fragments is also an important factor, generally the presence of isolated fragments does not harm the implantation capacity of the embryo, while the presence of numerous fragments can have a detrimental effect on the quality of the embryo. In embryos with a lot of fragmentation, what is known as "assisted hatching and fragments retrieval" can be done. This assisted hatching technique consists of slightly perforating the zona pellucida and aspirating the fragments through a pipette, thus improving communication between cells. It is only done in those embryos with high fragmentation, since it is difficult to perform and can be harmful to the embryo if it is not done correctly.
- Cell size and symmetry: Cell size is considered normal when the size of all blastomeras is similar, although there is usually a slight asymmetry in embryos. The presence of high asymmetry, with cells that differ between themselves 20% of the total volume, can be considered of poor prognosis, reducing the quality of the embryo to type C embryo. On the other hand, a number of odd cells, although all of them are of the same size, is considered asymmetric.
- Multinucleation:Multinucleation, that is, the presence of one or more nuclei within a cell may be caused by an error in cell division, fragmentation of the nucleus or incorrect migration of chromosomes during anafase. Embryons can show multinucleated blastomeras both in vitro and in vivo trials. The absence of multinucleation correlates with a high implantation rate and vice versa. In this way, the presence of multinucleated blastomeras in day 2 is associated with low implantation capacity (type D hybrids); the presence of multinucleated blastomeras in day 2 and day 3 is correlated with suboptimal embryos (type C); and finally, the emergence of multinucleation in day 3 does not affect both the implantation capacity. The existence of multinucleated blastomeras is related to mosaic and aneuploid embryos.
- Cytoplasm aspect: In the aspect of cytoplasm we can evaluate different parameters such as the presence of vesiculation, vacuolas and acytoplasmic rings. Generally during the first days of development cytoplasm presents a clear aspect, while on the third day the activation of the embryonic genome takes place, appearing vesicles that originate a granulate aspect. This change determines the correct development of the embryo but has a great relevance to distinguish the implantative capacity of an embryo. On the other hand, the appearance of vacuolas and the contraction of cytoplasm are correlated with the degeneration and lysis of the embryo. So embryos that present these alterations in more than two blastomeras are considered abnormal (type D). The cultivation of these embryos is usually extended to blastocyte to observe their evolution.
- Hairy zone: The pelvic area is a layer of 15 to 20 μm thick that surrounds and protects the mature egg. The presence of structural or functional anomalies in glycoproteins that form the pelvic area can cause problems such as the decrease in embryo viability and the decrease in implantation capacity. The thickness of the pelvic area is determinant for both the viability of the embryo and its ability to chloride. Thus if the pelvic area is thin, the embryo can easily chloride, increasing the implantation capacity; but it must be borne in mind that if it is very thin it may not adequately protect the embryo. On the contrary, if the pelvic area is very thick or has partitions it is difficult to hatch and implant the embryo. In the latter case, assisted hatching can be used.
Embryo transfer
The embryos are scored by the embryologist according to the number of cells, the growth parity, the degree of fragmentation, the state of the cytoplasm... Normally, to improve the chances of implantation and pregnancy, several embryos are transferred simultaneously. The number of embryos that are transferred depends on the number available, the age of the woman, diagnostic considerations and legal limitations (in some countries, the maximum number is limited to two or three, in Spain a maximum of 3 embryos can be transferred). The embryos that are considered "best" they are transferred to the woman's uterus through a very fine plastic cannula, which is inserted through the vagina and cervix and is controlled by ultrasound or echo-guided visualization. The cannula can be flexible, which is more expensive but it is the most recommended since it reduces the damage when it is inserted through the vagina until it reaches the uterus. Or rigid cannula, cheaper but less effective. Care must be taken to stimulate the uterus when performing the transfer. If the uterus is punctured with the cannula, it can lead to contractions of the uterus, which are detrimental to the implantation of the embryo after the transfer. Therefore, the use of Pozzi forceps or any instrument that punctures or attacks the cervix is not recommended, since it causes contractions that are harmful to the pregnancy. To reduce the risk of contractions, the woman receiving the embryos is administered progesterone, which is a hormone that relaxes smooth muscle and prevents contractions.
Success Rates
In the US, the live birth rate via IVF is around 27% per cycle (with a 33% pregnancy rate), but the chances of success vary greatly depending on the woman's age (or older). specifically, the age of the oocytes used).[11] When the woman's own oocytes are used (and not from a donor), for women under 35 years of age the pregnancy rate is around 43% per cycle (36.5% of live births), while for women by above 40 the rate drops drastically, to just 4% for women over 42 years.[12] Other factors that determine the success rate include the quality of the oocytes and sperm, the health of the uterus, and the experience of the clinic. Normally several embryos are transferred simultaneously, to improve the success rate, which has as a counterpart the risk of multiple pregnancy.
A recent technique involves immersing an embryo in a nutrient culture for 5 days until it reaches the blastocyst stage. Doctors then determine which embryos are most likely to develop. The best quality ones are transferred to the woman's uterus. In this way it is possible to improve the pregnancy rate without increasing the risk of multiple pregnancy. This is a relatively new technique and is in the experimental phase. The American Association for Reproductive Medicine (ASRM) believes that there is already sufficient scientific evidence to show that blastocyst transfer is the best option in patients with a good prognosis. ASRM recommends single embryo transfer to minimize the chance of having a multiple pregnancy.
Clinics with IVF programs generally publish their pregnancy rates. However, it is difficult to make comparisons between clinics, since the results are the consequence of many variables. In addition, the results also depend a lot on the type of patients selected.
There are many reasons why you may not get pregnant after IVF treatment and embryo transfer, including:
- The timing of ovulation may have been misinterpreted, or may not be predicted, or may not occur.
- Attempts to get oocytes that develop during the controlled cycle may not succeed.
- Obtaining oocytes may be abnormal or may have been damaged during extraction.
- A suitable sample of semen may not be available.
- Fertilization of oocytes to generate embryos may not occur.
- Cell division of fertilized oocytes may not take place.
- The embryo may not develop normally.
- Implantation may not take place.
- Failures with equipment, infections or human errors or other unforeseen and uncontrollable factors, which may result in loss or damage of oocytes, semen sample or embryos
According to a 2005 Swedish study published in the Oxford journal 'Human Reproduction', 166 women were monitored starting one month before their IVF cycles, and the results showed no significant correlation. between IVF outcomes and psychological stress. The study concluded with a recommendation to clinics that if IVF patients were informed of the results of the study, it might be possible to reduce the stress experienced during the treatment protocol. Although the psychological stress experienced during a cycle may not affect the outcome of IVF, it is possible that the IVF experience may result in stress that increases the chances of depression. The financial consequences of IVF alone (if a private clinic is used) can be anxiety-provoking and overwhelming. However, for many couples the alternative is infertility, and the experience of infertility itself can also cause stress and depression.
Complications
The major complication of IVF is the risk of multiple pregnancy.[13] This is directly related to the practice of transferring multiple embryos to increase the pregnancy rate. Multiple pregnancies are associated with an increased risk of miscarriage, obstetric complications, preterm birth, and neonatal morbidity with the possibility of long-term harm. In many countries there are strict limits to the maximum number of embryos that can be transferred, to reduce the risk of multiple pregnancy (triplets or more). Spontaneous division of the embryo in the uterus (as in a natural pregnancy) can also occur, but this is a rare case, generating identical twins. A double-blind randomized clinical trial followed IVF pregnancies resulting in 73 babies (33 boys and 40 girls) and concluded that 8.7% of singletons and 54.2% of twins had a birth weight < 2500 gr. In cycles where two embryos are transferred, the probability of having a twin pregnancy is 6%. In cycles where three embryos are transferred, the probability of having a twin pregnancy is 12% and of having a triple pregnancy is 3%.
Another risk of ovarian stimulation is the development of ovarian hyperstimulation syndrome, with a risk for the patient of less than 1%.
If the underlying infertility problem is related to abnormalities in spermatogenesis, the male offspring may be at higher risk for the same problem.
Defects related to Epigenetics
Epigenetics is defined as the study of the mechanisms that regulate the expression of genes without a modification in the DNA sequence. The epigenetic marks define the developmental capacity of the zygote and promote differentiation towards different cell types.
All in vitro fertilization techniques have consequences on epigenetic marks that can lead to infertility problems, risks to the survival of the fetus or phenotypic effects on the embryo.</23>
Using a mouse model, they simultaneously compared natural conception and gestation; naturally conceived blastocysts that were transferred to pseudopregnant (ET) recipients; blastocysts conceived in vivo after superovulation that were transferred to pseudopregnant (SET) recipients and IVF procedures, including superovulation, IVF, and culture of embryos to the blastocyst stage prior to ET. The findings demonstrate that even minimal in vitro manipulation such as NSET can affect placental development. Importantly, as the number of manipulations increases, the morphology and molecular phenotype of the placenta becomes more severe. </24>
The placenta [placenta] is known for its remarkable plasticity compared to other organs; it is capable of responding to changes caused by genetic disorders and environmental stressors through epigenetic mechanisms, including DNA methylation. IVF-induced epigenetic changes persist in tissues such as the brain and liver are the most affected and derive from the ectoderm and endoderm, respectively, indicating that the alterations occurred at an early stage of development, from the differentiation of the trophectoderm.
The prenatal period is a critical window of development. The phenotypes observed in this study, namely low birth weight and abnormal placentation, are certainly implicated in the etiology of cardiovascular and metabolic diseases, and further investigation into the long-term health effects of therapy is warranted..</24>
Defects in babies
The issue of the presence of defects associated with the IVF technique remains controversial. Most studies show that there is no significant increase after IVF, while others do not support this fact.
Some researchers believe that handling gametes and embryos outside the body could stimulate genetic changes (mutations) that can manifest as congenital defects at birth. Although there is no genetic evidence to support this idea, some epidemiological studies suggest a possible connection between assisted reproduction and rare genetic syndromes in newborns, such as Beckwith-Wiedemann syndrome, which is characterized by premature birth, a larger than normal tongue, and increased susceptibility to tumors and respiratory and speech defects. syndrome is rare, affecting only 1 in 12,000 newborns worldwide, but some studies suggest that it is more common in children born with assisted reproductive techniques.
However, the absolute risk of having a baby with Beckwith-Wiedemann syndrome is low, so experts find it difficult to advise a couple with fertility problems not to pursue assisted reproductive technology. Some researchers suggest that potential risks might be reduced by avoiding certain invasive procedures when not strictly necessary, such as biopsies of implanted embryos, culturing embryos in the laboratory for longer than the minimum necessary, and using ICSI in absentia. of male fertility problems.
Cryopreservation
Cryopreservation of embryos
When multiple embryos are generated after IVF, patients may choose to freeze embryos that are not transferred to the woman's uterus. These embryos are kept frozen in liquid nitrogen for up to 5 years. As published in 2006, there were about 500,000 frozen embryos in the US.[14] The advantage is that patients who fail to conceive after the first cycle can try again using frozen embryos, without having to perform a complete IVF cycle again: they would only have to transfer these embryos, without going through stimulation again., extraction and fertilization. Or, in the case of patients who do get pregnant, they can keep them for a second subsequent pregnancy. The remaining embryos from IVF can be donated to other women or couples for reproduction or for research with them.
There are different techniques for cryopreserving (freezing) embryos, each with different chances of achieving survival. Currently, the most effective method is vitrification (survival of up to 98%) [15], which in turn is reflected in a possibility of up to 50% of pregnancy with frozen embryos, according to reports in the medical literature [16 ]. This technique is characterized by a fast freezing speed (-23,000 °C/min), which prevents the formation of blade-effect crystals that could damage the embryo.
If, despite everything, there are still cryopreserved embryos that, due to the time that has elapsed or for other reasons, are not going to be used for implantation, the two possible alternatives (which are normally regulated by strict laws) are donation for research and destruction. In the case of embryo donation for research, this must be carried out in accredited centers and on the basis of projects authorized by the corresponding authorities. Normally, post-fertilization deadlines are established for the research on the embryos and, once the research is finished, it is not allowed to carry out an embryo transfer with them. Research with embryos from IVF has so far enabled studies on stem cells, of great importance in understanding embryonic development and in advancing tissue regenerative therapies. As for the destruction of frozen embryos, it is considered the last alternative, at the explicit request of the parents, or when they do not want them for themselves and have not authorized donation to other couples or research on them. Both the use of embryos for research purposes and their destruction generate extensive ethical debates between supporters and opponents, which translate into laws that limit the existing possibilities, which vary widely depending on the country.
In Spain, the period that the law requires to keep embryos frozen is five years. It will be at this time when the clinic that has the frozen embryos should contact via letter to request the actions to be carried out with the embryos. After 5 years have passed, and without having received a response from the owners of the frozen embryos, the clinic responsible for the embryos will be able to use them for the 3 causes mentioned above.
Cryopreservation of oocytes
Cryopreservation of unfertilized mature oocytes has been carried out successfully, for example in women who have a high probability of losing their oocyte reserves due to undergoing chemotherapy.
In a study with donors, there were no significant differences between the use of fresh oocytes and vitrified oocytes (type of cryopreservation). The fertilization rate was, respectively, 80.7 and 78.2%; the appearance of good quality embryos were 54.1 and 49.8%, those of implantation 33.3 and 34.0% and the percentages of babies born per cycle were 38.4 and 43.4%. That is, the studies showed equivalences in implantation, pregnancy production and gestation continuation between vitrified oocytes and fresh oocytes. In a second study using their own oocytes, the results of five clinical trials were analyzed, comparing fertilization, embryo quality, pregnancy production, and gestation continuation from 4,282 vitrified oocytes and 3,524 oocytes. fresh. The data were not different in the two groups and the survival rate of vitrified oocytes was 93%. In 2014, a meta-analysis included 21 prospective studies and concluded that the efficiency of oocytes was 7%, similar to that estimated for fresh oocytes. Furthermore, no differences were seen between oocytes that had been frozen for less than 6 months and those that had been frozen for more than 5 years. In fact, the longest storage of an egg that resulted in a baby has been 14 years.
Cryopreservation of ovarian tissue
Cryopreservation of ovarian tissue is aimed at patients who are going to undergo aggressive chemotherapy treatments that can destroy their reproductive tissues, thus causing their infertility. So, although today these treatments are a great advance in survival, they are not in quality of life, which is why it was necessary to develop such a technique. Technique that is still under study due to its complexity.
This tissue has a double physiological component, the endocrine part and the reproductive part. Therefore, its legislative regulation is complex. Everything related to it is adequately regulated by the National Transplant Organization. The material must be obtained prior to the toxic treatment. The material obtained must have a thin thickness to be able to spread the cryoprotective solution.
The most worrying thing is the risk of residual disease that could be reinserted in the patient. For this we have different in vitro diagnostic techniques that allow us to analyze the tissue in order to find remains of said residual disease. When performing the return transplant, follicular loss is worrisome. Therefore, a granulation tissue is being created that favors angiogenesis. All this is in the improvement phase but without a doubt it would be a great advance for society and for medicine.
Associated interventions
There are some variations or enhancements to IVF, such as ICSI, IMSI, Pronuclear Transfer, MST, ZIFT, GIFT, and PGD.
ICSI
Intracytoplasmic sperm injection (ICSI) is a recent development associated with IVF that allows a sperm to be directly injected into the oocyte's cytoplasm using micromanipulation techniques. It is used when the spermatozoa have difficulties penetrating the oocyte, and in that case the partner's or donor's sperm can be used. ICSI is also used when the sperm count is very low.
Intracytoplasmic Morphologically Selected Sperm Injection (IMSI)
IMSI or Intracytoplasmic Morphologically Selected Sperm Injection is an in vitrofertilization technique i> (IVF). It consists of carrying out a morphological selection of the spermatozoa before injecting them into the oocytes.
A spermatozoon is selected using an inverted microscope with a magnification of more than 6000 times its size in order to observe more precisely the composition of the sperm head, detecting possible abnormalities of the spermatozoa. vacuoles or damage to the DNA chain of the spermatozoa and choosing only those that do not present abnormalities to proceed with fertilization with the oocytes.
Assisted Hatching
Prior to transfer, embryos are enveloped in a layer of glycoproteins, and to achieve a pregnancy, embryos must break out of this envelope before implanting. It is involved called the zona pellucida and assisted hatching consists of making a hole in this area to facilitate the hatching process of the embryo and thus increase the implantation rate.
Assisted hatching (also called AHA) is a technique that has been used since the 1980s, when it was found that PZD (partial zona pellucida dissection) embryos did appear to have a higher implantation rate than embryos. normal embryos. This method can be done on any development day, although it's usually done on the third day.
Although it is a process that also occurs physiologically, there are certain reasons why an embryo is unable to carry out the natural hatching process, among which are:
- A large percentage of fragmentation in the embryo.
- Very hard or unsuitable pelvic area.
On these occasions is when assisted hatching processes are used, which may or may not be accompanied by the aspiration of cell fragments, depending on the aforementioned cause. However, there are other reasons why it can be carried out, such as a preimplantation diagnosis. In this case, it is usually done as a previous step.
There are different ways to do Assisted Hatching, either by the chemical, mechanical or laser method. The latter is the one that has gained the most popularity due to its good results. Chemical method: consists of introducing Thyroid acid, which is a buffered saline solution with a pH of 2.5 (with a margin of error of 0.3), into an AHA pipette. The embryo, on the other hand, is held in place with a holding micropile and in a 20 microliter HEPES solution. The pipette with Tyrode's solution is approached and released in the vicinity of the embryo. The zona pellucida degrades, since the pH is so low, the proteins that form it are denatured, giving rise to a hole in that zone. Once formed, this solution is aspirated with the pipette so that it does not affect the interior of the embryo, and we move the embryo away from this area. The advantage of this method is that it is inexpensive and the ends are smooth. The drawbacks of this method are: the embryo is exposed to an acid solution, increasing the risk of damage to it and, in addition, the opening is permanent, which can be harmful as it affects the internal environment of the embryo.
Mechanical method: this method is carried out in 20 microliters of medium with HEPES and consists of fixing the embryo with a holding pipette, on the other hand, a pipette (PZD) is taken with which it is tangentially traverses the zona pellucida. Once the embryo has passed through, it is released and the area is torn against the holding pipette. In this case, as a final result, we obtain a buttonhole that remains closed and through which the embryo will later be easy to come out and implant. The eyelet may have dimensions of approximately 50 microns. The advantages of this method are: that the embryo is more protected by the effect of the buttonhole, it is more natural than the other methods and it is also cheaper. On the other hand, its drawback is: that it is difficult to learn, as well as laborious.
Physical method (laser) : this method is performed in a medium with HEPES where the embryo is located. The infrared diode laser (1.48 micron) is aimed and fired, through the objective, close to where the embryo is located. The laser action time can be varied (0.1 to 50 ms). The laser locally heats the water, and as the temperature increases, the proteins that make up the zona pellucida close to the laser become denatured, thus creating a hole. The shape of the laser hole is an open eyelet always larger than that seen in the plane of the microscope. The advantage of this method is that it is very fast as well as being reproducible. On the other hand, its disadvantages are: it is very expensive, the embryo is exposed to a risk such as the laser itself, and the opening is permanent, in addition to being larger than expected. that it seems and this, as we have said before, can affect the embryo.
It must be said that this technique only offers advantages in the following cases: -Women older than 37 years. -Pregnancy failure after IVF/ICSI.
and other proposed but not yet proven indications: -Abnormal zona pellucida. -Poor embryo quality. -Low ovarian response.
Finally, it is appropriate to say that the total hatching of the embryo has also been attempted through the chemical method or with pronase. It is only possible to perform it at the blastocyst stage, since if it were done earlier the embryo would disintegrate into its blastomeres. But this technique is rarely used in the laboratory.
Nuclear or pronuclear transfer
In nuclear or pronuclear transfer (pronuclear transfer), the pronuclei of a recently fertilized oocyte, both maternal and paternal, are taken and transferred to a previously enucleated oocyte from a donor. The donor oocyte, therefore, will be the one that provides all the tools for cell division, as well as the organelles. In this way it is achieved that all the nuclear DNA (chromosomal genetics) of the embryo comes from both parents, being only the mitochondrial DNA of the donor.
Nuclear transfer is not legal in many countries, however it offers hopeful clinical application for women with mitochondrial diseases or older women who wish to father healthy children.
Spindle Transfer (MST)
In the spindle transfer (maternal spindle transfer or MST) oocytes are taken in metaphase II, the last phase of oocyte maturation prior to fertilization. At this stage, the chromosomes are arranged in a 'spindle complex' or spindle complex aligned. In this technique the 'spindle' from the patient to an oocyte from a previously enucleated donor. In this way, as in the prior art, it is possible to avoid any disease associated with mitochondrial DNA, because although the transferred oocyte would preserve the patient's nuclear DNA; the mitochondrial would come from the donor.
What differentiates this technique from the previous one is that it would be performed prior to fertilization with the sperm.
Intrafallopian transfer of zygotes
In zygote intrafallopian transfer (ZIFT), oocytes are removed from the woman, fertilized in vitro, and the embryos are placed in the fallopian tubes. fallopian, rather than in the uterus.
TGIF
In GIFT, the oocytes are removed from the woman and placed in one of the fallopian tubes, along with the man's sperm. Therefore, this variation is actually in vivo fertilization and not in vitro.
EGP (PGT)
The EGP Preimplantation Genetic Study) can be performed on the embryos prior to transfer. A similar but more general test is preimplantation genetic haplotyping or HGP (PGH in English). However, the success rate of PGD is low.
Mini IVF
Originally developed by the New Hope Fertility groups and the Kato Ladies Clinic, [17] [18] [19] Mini IVF has the particularity of stimulating the ovary in a very subtle way with the minimal use of hormonal medications. The rest of the stages in the Mini IVF techniques are similar to those of traditional IVF, although with minor changes aimed at reducing the risk and discomfort for patients. This technique is recommended for women with a good prognosis, patients who cannot receive complete ovule stimulation or for women who do not wish to receive a more aggressive hormonal treatment. Currently, and according to the American Society for Assisted Reproduction (ASRM), Mini-IVF is considered the protocol of choice for patients with low ovarian reserve, and poor responders. The challenges involved in working with a smaller number of follicles demand that Mini-IVF be performed by a team with extensive experience in this technique, and in state-of-the-art laboratories.
History
The first pregnancy achieved through IVF with a human oocyte was described by Monash's team in The Lancet in 1973, although it only lasted a few days and today would be called a biochemical pregnancy. A tubal ectopic pregnancy was then published by Steptoe and Edwards in 1976. In 1978, Edwards and Steptoe achieved the first IVF birth. The girl Louise Brown was born on 25 July 1978 at the Royal Oldham Hospital in Lancashire, Lancashire. near Manchester (United Kingdom) and another unknown baby, the first IVF babies.[citation needed] Robert G. Edwards received the Nobel Prize in Physiology and Medicine 2010 for the development of in vitro fertilization'.
Then came the birth of Candice Reed in Melbourne in 1980. The use of clomiphene citrate-stimulated cycles and the use of human chorionic gonadotropin (hCG) to control the timing of oocyte maturation, thus allowing controlling the timing of extraction turned IVF from a research tool into a clinical treatment.
14 pregnancies followed, followed by 9 births in 1981 with the Monash varsity team. Jones's team in Norfolk, Virginia improved stimulation cycles by incorporating the use of a follicle-stimulating hormone (uHMG). This became known as controlled ovarian hyperstimulation (COH). Another step forward was the use of gonadotropin-releasing hormone agonists (GnRH-A), thus decreasing the need for control by preventing premature ovulation, and more recently gonadotropin-releasing hormone antagonists (GnRH-Ant), with a similar function. The additional use of oral contraceptives has allowed IVF cycles to be scheduled, making the treatment easier for doctors and patients to perform.
In Clínica 2200 the first in vitro fertilization in Spain was performed in April 1984, by gynecologists Pedro Barri and Angel Sopeña, and doctor Marisa Lopez Tapia.
The first IVF baby in Latin America was born in Colombia in 1985, in the laboratory of Dr. Elkin Lucena thanks to scientific collaboration with experts from Madrid. The same team is also responsible for the first embryo freezing pregnancies in Latin America.
The ability to freeze and subsequently thaw and transfer embryos has also significantly improved the effectiveness of IVF. Another significant moment was the development of intracytoplasmic sperm injection (ICSI) by Gianpiero Palermo in Brussels in 1992. This has enabled men with minimal sperm production to achieve pregnancies, sometimes in conjunction with sperm retrieval, using a testicular needle. fine or open testicular biopsy, so even men with Klinefelter syndrome can sometimes achieve a pregnancy. Thus, IVF has become the solution to most infertility problems, from tubal problems to male factors, idiopathic subfertility, endometriosis, advanced maternal age, and anovulation.
Additional bibliography
- George, Robert P.; Tollefsen, Christopher (2012). Embryo: a defense of human life. Rialp Editions. ISBN 9788432142345.
- Andorno, Roberto (1992). «In vitro fertilization of the distinction between people and things». Person and law: Revista de substantiateción de las instituciones Jurídicas y de Derechos Humanos (26): 9-27. Consultation on 9 December 2015.