Immune system
The immune system or immunological system is the set of biological elements and processes within an organism that allows it to maintain homeostasis or internal balance in the face of external aggressions, whether of a biological nature (pathogens) or physicochemical (such as pollutants or radiation) and internal (for example, cancer cells). Recognize what is harmful and react to it (whether it is an external or internal aggression).
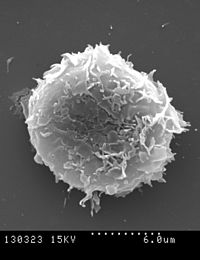
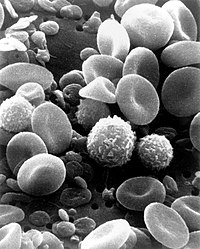
The immune system is made up of soluble molecules (such as complement system proteins, antibodies, histamine, etc.) in different fluids (blood and lymph, among others) and cells located in different tissues and organs, mainly: bone marrow, thymus, spleen, lymph nodes, and MALT or mucosa-associated lymphoid tissue. Different types of leukocytes or white blood cells are generated in the bone marrow, which are cells specialized in immune function: neutrophils, lymphocytes, eosinophils, basophils, mast cells, monocytes, dendritic cells, and macrophages; all of them move through the blood and the lymphatic system to the different organs.
There are different levels to the immune response. Some response elements are invariant over time and are grouped into the so-called innate immune response (natural or nonspecific) and alternatively the elements capable of memorizing microorganisms are organized into the acquired immune response (adaptive or specific). Innate response elements are present in virtually all living things, even simple single-celled organisms such as bacteria have enzyme systems that protect them against viral infections. Other basic immune mechanisms are found in eukaryotes, plants, fish, reptiles, and insects, as well as mammals. These mechanisms include antimicrobial peptides called defensins and cytokines, phagocytosis by neutrophils and macrophages, the complement system, and others. The innate immune system can detect in cells a variety of “danger” signals called danger-associated molecular patterns (DAMPs) or the presence of pathogen-associated signals called pathogen-associated molecular patterns (PAMPs)., for its acronym in English), thus identifying a wide variety of damaged cells, whether by burns, radiation, viruses, bacteria, parasites and many other agents, distinguishing them from healthy cells and tissues of the organism to function properly.
The acquired immune response allows vertebrates, such as humans, to have more sophisticated defense mechanisms, interconnected with the mechanisms of the innate immune system in a dynamic and longer-term manner. The anatomical-functional unit of this system is the lymphocyte. The immune system adapts over time to recognize specific pathogens more effectively, generating an immune memory. The immune memory created from a primary response to a specific pathogen provides an enhanced response to secondary encounters with that same specific pathogen. This process of acquired immunity is the basis of vaccination.
Disorders in the immune system can cause many diseases. Immunodeficiency occurs when the immune system is less active than normal, leading to recurrent and life-threatening infections. Immune deficiency may be the result of a genetic disease, such as severe combined immunodeficiency, or be caused by drugs or an infection, such as acquired immunodeficiency syndrome (AIDS) which is caused by the retrovirus HIV. In contrast, the diseases Autoimmune disorders are the result of an overactive immune system that attacks normal tissues as if they were foreign organisms. Common autoimmune diseases include Hashimoto's thyroiditis, rheumatoid arthritis, type 1 diabetes mellitus, and lupus erythematosus. Immunology covers the study of all aspects of the immune system that have significant relevance to human health and disease. It is expected that further research in this field will play an important role in promoting health and treating diseases.
Terminology
The word “immunity” is a 19th-century neologism derived from the Latin in-mūn(itātem) 'without obligation', whose current meaning dates back to 1866, probably influenced by the Latin military term immunīre 'defend from within'.
The related terms «immunological» (of or relating to immunity), «immunological» (of or relating to immunology), «immunology» (the study of biological immunity and its applications) and «immune» (not attackable by certain diseases; or, pertaining to or related to the causes, mechanisms or effects of immunity), are all words accepted by the RAE.
Regarding academic use, in accordance with the definitions of the RAE, it is correct to refer to both immune system and immune system, since in the latter case the The word immune takes the meaning of "belonging or relating to the causes, mechanisms, or effects of immunity" (Note: in English "immune" is used for both immune and immune). However, the use of immunological system can be observed in numerous publications and reference works in the Spanish language.
Primary and secondary organs
The immune system consists of a series of organs, tissues, and cells widely distributed throughout the body. Functionally, the organs are classified into primary and secondary. The primary ones are the bone marrow and the thymus, which are the ones that provide the microenvironment for the maturation of the lymphocytes of the acquired immunity. The secondary organs are the lymph nodes, the spleen, the lymphoid tissue associated with the mucous membranes (and partly the bone marrow itself) where the immune cells migrate with the microorganism or antigen inside, providing the appropriate environment for the lymphocytes. interact with it, in the process called antigen presentation.
Immune lines of defense
The immune system protects organisms from infection with several lines of defense of increasing specificity. The first line of defense is constituted by the physical, chemical and biological barriers that are the skin and all the mucosal surfaces of the organism (gastrointestinal, respiratory, genitourinary tract, as well as the ocular conjunctiva), which prevent pathogens such as bacteria and viruses from entering the organism. If a pathogen penetrates these barriers, innate immunity offers an immediate but non-specific response. Innate response elements exist in all plants and animals. However, if pathogens evade the innate response, vertebrates possess a third level of defense, which is the acquired immune response. Here the immune system adapts its response during infection to improve recognition of the pathogen. Information about this enhanced response is retained even after the pathogen is eliminated, in the form of immune memory, and allows the adaptive immune system to unleash faster and stronger attacks if the immune system detects this type of attack in the future. pathogen.
Characteristics of the immune system
| Immunity Innata | Adaptive immunity |
| The answer is not specific. | Specific response. |
| It responds to Molecule Patterns Associated with Pathogens (PAMPs). | It responds to pathogen antigens. |
| Exposure leads to the immediate maximum response. | Delay between exposure and maximum response. |
| Immunity mediated by cells and humoral components. | Immunity mediated by cells and humoral components. |
| No immune memory. | Exposure leads to immune memory. |
| Present in almost all life forms. | Present only in mandibulous vertebrates. |
Both innate and adaptive immunity depend on the immune system's ability to distinguish between self and non-self molecules. In immunology, self molecules are those components of an organism that the immune system distinguishes from foreign substances. Conversely, molecules that are not part of the organism are recognized as foreign molecules. One type of foreign molecules are called antigens ('anti', from the Greek Δντι- which means 'opposite' or 'with contrary properties' and 'geno' #34;, from the Greek root γεν, to generate, to produce [generating or creating opposition]), are substances that bind to specific immune receptors and trigger an immune response.
Innate immunity
Microorganisms or toxins that manage to enter an organism will encounter the cells and mechanisms of the innate immune system. The innate response is usually triggered when microbes are identified by pattern recognition receptors, which recognize components that are present in broad groups of microorganisms, or when damaged, injured, or stressed cells send out alarm signals, many of which (but not all) are recognized by the same receptors that recognize pathogens. Germs that manage to penetrate an organism will encounter the cells and mechanisms of the innate immune system. The defenses of the innate immune system are non-specific, which means that these systems recognize and respond to pathogens in a generic way. This system does not confer long-lasting immunity against the pathogen. The innate immune system is the dominant protective system in the vast majority of organisms.
Surface and chemical barriers
Various barriers protect organisms from infection, including mechanical, chemical, and biological barriers. The ceruminous cuticles of many leaves, the exoskeleton of insects, the shells and membranes of externally laid eggs, and the skin are examples of mechanical barriers that form the first line of defense against infection. Organisms cannot be completely isolated from their environment, other systems participate in the protection of body openings, such as the lungs, intestines and the genitourinary system. Lungs, coughs, and sneezes mechanically expel pathogens and other irritants from the airways. The cleansing action of tears and urine also expels pathogens mechanically, while mucus secreted by the respiratory and gastrointestinal tracts serves to trap and engage microorganisms.
Chemical barriers also protect against infection. The skin and respiratory tract secrete antimicrobial peptides such as β-defensins. Enzymes such as lysozyme and phospholipase A in saliva, tears, and breast milk are also antibacterial agents. Secretions from the vagina serve as chemical barriers at menarche, when they become slightly acidic, while semen contains defensins and zinc to kill pathogens. In the stomach, gastric acid and peptidases act as powerful chemical defenses against ingested pathogens.
Within the genitourinary and gastrointestinal tracts, the commensal microbiota serves as a biological barrier by competing with pathogenic bacteria for food and space, and in some cases modifying environmental conditions, such as pH or available iron content. This reduces the probability that the population of pathogens will reach a sufficient number of individuals to cause disease. However, since most antibiotics do not discriminate between pathogenic bacteria and normal flora, oral antibiotics can sometimes cause fungal overgrowth (fungi are not affected by most antibiotics) and lead to conditions such as vaginal candidiasis (caused by yeast). Reintroduction of probiotic microorganisms, such as lactobacillus, found in yogurt, help restore a healthy balance of microbial populations in intestinal infections in children, and there are also encouraging preliminary data from studies on bacterial gastroenteritis, inflammatory bowel diseases, urinary tract infections, and post-surgical infections.
Humoral barriers
Fever
Fever, defined as a rise in body temperature above 37.7°C, is actually a protective response to infection and injury, considered a stimulation of the body's immune system. Fever is caused by a type of monocytes known as pyrogens, which are natural substances that cause fever, forcing the body to produce its own as a defense against any possible infection. However, infections do not are the only cause of fever, often it may not be an immune response.
Usually, a fever has an obvious cause such as an infection caused by a virus, bacteria, fungus or protist, some type of cancer, an allergic reaction, hormonal disturbances, excessive exercise, autoimmune diseases, damage to the hypothalamus (gland). endocrine in charge of regulating body temperature; it is like a thermostat) or due to excessive exposure to the sun. Fever, due to its potential beneficial effects, is debated whether it should be routinely treated. Fever benefits the immune system to more efficiently combat "invaders": increasing and improving the motility and phagocytosis of leukocytes, lowering endotoxin levels, increasing T-cell proliferation, and improving interferon activity. Fever may follow a pattern in which it reaches a maximum daily temperature and then returns to its normal level. Similarly, the fever can be remittent, that is, the temperature varies, but does not return to normal.
Inflammation
Inflammation is one of the immune system's first responses to infection. Symptoms of inflammation are redness, swelling, and warmth. The redness is caused by increased blood flow in the affected tissue, the swelling is due to the accumulation of immune system cells, which in turn release different substances to attack the pathogen and to continue recruiting other leukocytes. And the heat is generated by the metabolic process of the cells that are in action.
Inflammation is produced by eicosanoids and cytokines, which are released by injured or infected cells. Eicosanoids include prostaglandins which produce fever and blood vessel dilation associated with inflammation, and leukotrienes which attract certain leukocytes. Cytokines include interleukins which are responsible for communication between leukocytes; chemokines that promote chemotaxis; and interferons which have anti-viral effects such as suppression of protein synthesis in the host cell. Growth factors and cytotoxic factors may also be released. These cytokines and other chemicals attract immune cells to the site of infection and promote healing of damaged tissue by removing pathogens.
Plugin system
The complement system is a biochemical cascade that attacks foreign cell surfaces. It contains more than 20 different proteins and is named for its ability to complement antibody-initiated destruction of pathogens. The complement system is the major humoral component of the innate immune response. Many species have complement systems, not only in mammals, but also in plants, fish and some invertebrates.
In humans, this response is activated by the binding of complement proteins to carbohydrates on the surfaces of microorganisms or by the binding of complement to antibodies that have in turn bound to the microorganisms. This recognition signal produces a rapid killing response. The speed of the response is the result of signal amplification that occurs upon sequential proteolytic activation of complement molecules, which are also proteases. Upon initial binding of complement proteins to the microbe, they activate its protease capacity, which in turn activates other complement proteases, and so on. This produces a catalytic cascade that amplifies the initial signal through controlled positive feedback. The cascade results in the production of peptides that attract immune cells, increase vascular permeability, and opsonize (coat) the surface of the pathogen, marking it for destruction. This complement deposition can also kill cells directly by blocking their plasma membrane.
Cellular barriers of the innate system
Leukocytes (white blood cells) act as independent single-celled organisms and are the second arm of the innate immune system. Innate leukocytes include phagocytes (macrophages, neutrophils, and dendritic cells), mast cells, eosinophils, basophils, and killer cells. natural. These cells identify and eliminate pathogens, either attacking larger ones through contact or engulfing others to kill them. Innate cells are also important mediators in the activation of the adaptive immune system.
Phagocytosis is an important feature of cellular innate immunity, carried out by cells called phagocytes, which engulf or eat pathogens and particles, surrounding them on the outside with their membrane until they pass into their cytoplasm. Phagocytes generally patrol in search of pathogens, but can be attracted to specific locations by cytokines. Upon being engulfed by the phagocyte, the pathogen becomes engulfed in an intracellular vesicle called a phagosome which then fuses with another vesicle called a lysosome to form a phagolysosome. The pathogen is destroyed by the activity of the digestive enzymes of the lysosome or as a consequence of the so-called "respiratory jet" which releases oxygen free radicals into the phagolysosome. Phagocytosis evolved as a means of acquiring nutrients, but this role was extended in phagocytes to include the engulfment of pathogens as a defense mechanism. Phagocytosis probably represents the oldest form of host defense, as it has been identified in vertebrate and invertebrate animals.
Neutrophils and macrophages are phagocytes that travel through the body in search of invading pathogens. Neutrophils are normally found in the blood and are the most common type of phagocytes, typically accounting for 50-60% of the total number of phagocytes. leukocytes that circulate in the body. During the acute phase of inflammation, particularly in the case of bacterial infections, neutrophils migrate to the site of inflammation in a process called chemotaxis, and are the first cells to arrive on the scene. of the infection. Macrophages are versatile cells that reside within tissues and produce a wide range of substances including enzymes, complement proteins, and regulatory factors such as Interleukin 1. Macrophages also act as scavengers, ridding the body of dead cells and other debris., and as "antigen-presenting cells" to activate the adaptive immune system.
Dendritic cells are phagocytes in tissues that are in contact with the external environment; thus they are located primarily in the skin, nose, lungs, stomach, and intestines. They are so named because of their resemblance to neuronal dendrites, both of which have many spicular projections on their surfaces, but dendritic cells are not. in any way related to the nervous system. Dendritic cells act as a link between the innate and adaptive immune systems by presenting antigens to T cells, one of the key cell types of the adaptive immune system.
Mast cells reside in connective tissues and mucous membranes and regulate the inflammatory response. They are most often associated with allergy and anaphylaxis. Basophils and eosinophils are related to neutrophils. They secrete chemical mediators that are involved in defense against parasites and play a role in allergic reactions, such as asthma. Natural Killer (NK) cells are white blood cells that attack and destroy tumor cells, or cells that have been infected by viruses.
Adaptive or acquired immunity
The adaptive immune system evolved in primitive vertebrates and allows a greater immune response, as well as the establishment of the so-called "immunological memory", where each pathogen is "remembered" by a characteristic and self-antigen of that particular pathogen. The adaptive immune response is antibody-specific and requires recognition of non-self antigens during a process called "antigen presentation". Antigen specificity allows the generation of responses that are tailored to specific pathogens or cells infected by pathogens. The ability to mount these specific responses is maintained in the body by memory cells. If a pathogen infects an organism more than once, these memory cells trigger a response specific to that pathogen they have recognized, in order to quickly eliminate it.
Lymphocytes
Cells of the adaptive immune system are a special class of white blood cells, called lymphocytes. B cells and T cells are the major classes of lymphocytes and are derived from bone marrow pluripotent hematopoietic stem cells. B cells are involved in the humoral immune response, while T cells are involved in the immune response mediated by cells.
B cells and T cells contain receptor molecules that recognize specific targets. T cells recognize a non-self target, such as a pathogen, only after the antigens (small fragments of the pathogen) have been processed and presented in combination with a self receptor, a molecule called the major histocompatibility complex (MHC). There are two main subtypes of T cells: the killer T cell (CD8 T cell) and the helper T cell (CD4 T cell). Killer T cells only recognize antigens coupled to MHC class I molecules, while helper T cells only recognize antigens coupled to MHC class II molecules. These two mechanisms of antigen presentation reflect the different roles of the two types of T cells. A third minor subtype is formed by γδ T cells (gamma/delta T cells), which recognize intact antigens that are not coupled to MHC receptors.
In contrast, the B-cell antigen-specific receptor is an antibody molecule on the surface of the B cell, and recognizes whole pathogens without the need for antigen processing. Each lineage of B cells expresses a different antibody on its surface, such that the complete set of antigen receptors on an organism's B cells represents all the antibodies that organism is capable of making.
Cytotoxic T lymphocytes
Cytotoxic T lymphocytes, are a subset of T cells that kill cells infected with viruses (and other pathogens), or otherwise damaged or diseased. Like B cells, each type of T cell recognizes a different antigen. Killer T cells are activated when their T cell receptor (TCR) binds their specific antigen in complex with the MHC class I receptor of another cell. The recognition of this MHC-antigen complex is favored by a co-receptor on the T cell, called CD8 (hence its name T-CD8). Thus, the T cell travels through the body in search of cells where MHC class I receptors carry this antigen.
When an activated T cell comes into contact with such cells, it releases cytotoxins that form pores in the plasma membrane of the target or recipient cell, allowing ions, water, and toxins to enter it. This causes the target cell to burst or undergo apoptosis. Killer T cell-induced death of host cells is of great importance in preventing virus replication. T cell activation is tightly controlled and usually requires a very strong activation signal from the MHC/antigen complex, or additional activation signals provided by helper T cells (see below).
Help T cells
Help T cells regulate both the innate and adaptive immune responses, and help determine what type of immune response the body will mount to a particular pathogen. These cells do not have any type of cytotoxic activity and do not kill cells infected or kill pathogens directly. Instead, they control the immune response by directing other cells to carry out these tasks.
Help T cells express T cell receptors that recognize antigens bound to MHC class II molecules. The MHC-antigen complex is also recognized by the helper T cell CD4 coreceptor, which recruits molecules within the T cell (such as Lkc) that are responsible for T cell activation. Helper T cells have a weaker association with the MHC-antigen complex than do cytotoxic T cells, meaning that many receptors (about 200 to 300) of the helper T cell must bind to an MHC-antigen to activate the T cell. lymphocyte, whereas cytotoxic T lymphocytes can be activated by the coupling of a single MHC-antigen molecule. Activation of helpers also requires a longer duration of binding to an antigen-presenting cell. Activation of a resting helper T cell causes it to release cytokines that influence the activity of many cell types. Cytokine signals produced by helper T cells enhance the microbicidal function of macrophages and the activity of cytotoxic T cells. In addition, activation of helper T cells results in an increase in molecules that are expressed on the surface of the lymphocyte. T, like the CD40 ligand (also called CD154), which sends out additional stimulatory signals usually required to activate antibody-producing B lymphocytes.
γδ T cells
γδ T cells represent a small subpopulation of T cells characterized by having a different T cell receptor (TCR) on their surface. Most T cells have an RCT composed of two glycoprotein chains called α and β chains; however, in γδ T cells its receptor is made up of two chains called γ and δ. This group of T cells is, in general, less numerous than that of the αβ cells and it is in the mucosa of the intestine where they are found in greater numbers, forming part of a population of lymphocytes called "intraepithelial lymphocytes".
The antigenic molecules that stimulate γδ T cells are largely unknown, however, these cells are peculiar in that they appear to not require antigens to be processed and presented bound to MHC molecules, although some recognize MHC class IB molecules. On the other hand, it is believed that γδ T cells play a major role in the recognition of antigens of a lipid nature.
γδ T cells share the characteristics of helper, cytotoxic, and natural killer T cells. Like other non-conventional T cell subpopulations that carry invariant or constant TCRs, such as some subtypes of natural killer T cells, γδ cells lie on the borderline between innate and adaptive immunity. On the one hand, γδ cells form part of adaptive immunity because they are capable of reorganizing the genes of their RCTs to produce a diversity of receptors and develop a phenotypic memory, that is, being carriers of receptors adapted to specific antigens or pathogens. On the other hand, they are also part of the innate immune system, since the different subpopulations also have receptors capable of acting as pattern recognition receptors. Thus, for example, large numbers of human Vγ9/Vδ2 T cells (a common subtype of non-peptide cells produced by microorganisms, while another subtype of T cells, Vδ1 in epithelia, respond to epithelial cells carrying markers that have suffered some kind of stress.
Antibodies and B lymphocytes
The B cell identifies pathogens when antibodies on its surface bind to specific foreign antigens. This antigen/antibody complex passes into the B cell where it is processed by proteolysis and broken down into peptides. The B lymphocyte then displays these peptide antigens on its surface bound to MHC class II molecules. This MHC/antigen combination attracts a helper T cell that has receptors complementary to that MHC/antigen complex. The T cell then releases lymphokines (the type of cytokines produced by lymphocytes) and thus activates the B lymphocyte.
When the B lymphocyte has been activated, it begins to divide and its descendants secrete millions of copies of the antibody that recognizes that antigen. These antibodies circulate in blood plasma and lymph, bind to pathogens carrying these antigens, leaving them marked for destruction by complement activation or ingestion by phagocytes. Antibodies can also neutralize certain threats directly, by binding to bacterial toxins or by interfering with the receptors that viruses and bacteria use to infect cells.
Alternative Adaptive Immune System
Although the classic molecules of the adaptive immune system (for example, antibodies and T-cell receptors) exist only in jawed vertebrates, a different molecule, derived from lymphocytes, has been discovered in primitive jawless vertebrates, such as the lamprey and marine animals of the family Myxinidae. These animals possess a large variety of molecules called lymphocytic variable receptors (VLRs) which, like the antigen receptors of jawed vertebrates, are produced by a small number of genes (one or two). These molecules are thought to bind to pathogen antigens in a similar way to antibodies and with the same degree of specificity.
Immune memory
When B and T cells are activated and begin to replicate, some of their offspring will become long-lived memory cells. Throughout the life of a Homo sapiens, these cells will remember each specific pathogen they have encountered and can trigger a strong response if they detect that particular pathogen again. This is "adaptive" because it occurs during an individual's lifetime as an adaptation to infection by that pathogen and prepares the immune system for future challenges. Immune memory can be passive and short-lived or active and long-lived.
Passive immunity
Passive immunity is generally short-lived, from a few days to a few months. Newborns have not had previous exposure to microbes and are particularly vulnerable to infection. The mother provides them with several layers of passive protection. During pregnancy, a particular type of antibody, called IgG, is transported from mother to baby directly through the placenta, so human babies have high levels of antibodies from birth and with the same range of specificity against antigens as his mother. Breast milk also contains antibodies that, when they reach the baby's intestine, protect him from infections until he can synthesize his own antibodies.
This is all a form of passive immunity because the fetus doesn't actually make memory cells or antibodies, it just borrows them from the mother. In medicine, passive protective immunity can also be artificially transferred from one individual to another through antibody-rich serum.
Active immunity and immunization
Long-term active memory is acquired after infection, by activation of T and B cells. Active immunity can also be generated artificially, through vaccination. The principle behind vaccination (also called immunization) is to introduce an antigen from a pathogen to stimulate the immune system to develop specific immunity against that particular pathogen without causing the disease associated with that microorganism.
Almost all viral vaccines are based on "live" attenuated viruses, while bacterial vaccines are based on non-cellular components or fragments of bacteria, including harmless components of toxins. Since many vaccines derived from acellular antigens do not induce For a sufficiently strong adaptive response, adjuvants that activate antigen-presenting cells of the innate immune system are added to most bacterial vaccines to enhance immunogenicity.
This deliberate induction of an immune response is effective because it exploits the natural specificity of the immune system as well as its inducibility. Being the infectious disease one of the most frequent causes of death in the human population, vaccination represents the most effective manipulation of the immune system that humanity has developed. Although it should be clarified that this is not why people will not get sick.
In addition to providing protection to those who receive the vaccine, the act of immunization in the population generates a phenomenon known as "herd immunity" or "herd immunity." This effect is achieved from 95% of vaccinated people. Herd immunity helps protect the most vulnerable groups in society such as infants, the elderly, immunocompromised patients, and transplant recipients. Therefore, vaccinated people act as a barrier, and do not allow pathogenic microorganisms to reach the most defenseless, helping to eradicate diseases, most of which are deadly.
Human Immunity Disorders
The immune system is a remarkably efficient complex that incorporates specificity, inducibility, and adaptation. However, sometimes failures occur that can be grouped, generically, into the following three categories: immunodeficiency, autoimmunity and hypersensitivity.
Immunodeficiencies
Immune deficiency occurs when one or more of the components of the immune system become inactive. The ability of the immune system to respond to pathogens and diseases is reduced in both children and the elderly, and the immune response begins to decline after about fifty years of age, due to immunosenescence(it is a progressive decrease in the immune response that affects all components of the immune system). In developed countries, obesity, alcoholism, and drug use are common causes of poor immune function. However, malnutrition is the most common cause of immunodeficiency in developing countries. A diet lacking in insufficient proteins with deficiencies in cellular immunity, complement activity, phagocyte function, IgA antibody levels, and cytokine production. Deficiencies in specific nutrients such as iron, copper, zinc, selenium, vitamins A, C, E, and B6, and folic acid (vitamin B9) also reduce the immune response. In addition, loss of the thymus at an early age due to a genetic mutation or surgical removal results in severe immunodeficiency and high vulnerability to infection.
Immune deficiency can be inherited or acquired. Chronic granulomatous disease, in which phagocytes have a reduced ability to kill pathogens, is an example of inherited or congenital immunodeficiency. AIDS and some types of cancer cause acquired immunodeficiency.
Autoimmunity
Exaggerated immune responses span the other extreme of immune dysfunction, particularly autoimmune diseases. Here the immune system fails to adequately distinguish self from foreign and attacks parts of the body itself. Under normal circumstances, many T cells and antibodies react with the body's own peptides. There are, however, specialized cells (located in the thymus and in the bone marrow) that participate in the elimination of young lymphocytes that react against self antigens, to thereby preventing autoimmunity. Autoimmune reactions can be triggered in several ways:
- A body substance that normally covers a specific area and is released in the general circulation; and consequently is hidden in the immune system.
- The alteration of a body substance.
- The immune system responds to a strange substance—old—that seems to have the same characteristics to a natural substance of the body and involuntarily proceeds to attack both the substances of the body and the strangers.
- The malfunction of cells that control the production of antibodies.
Hypersensitivity
Hypersensitivity is an immune response that damages the body's own tissues. It is divided into four classes (Types I-IV) based on the mechanisms involved and the development time of the hypersensitive reaction. Type I hypersensitivity is an immediate or anaphylactic reaction, related to allergies. Symptoms range from mild discomfort to death. Type I hypersensitivity is mediated by immunoglobulin E, which is released by mast cells and basophils. Type II hypersensitivity occurs when antibodies bind to antigens located on the patient's own cells, marking them for destruction. It is also called antibody-dependent or cytotoxic hypersensitivity and is mediated by IgG and IgM antibodies. Immune complexes (aggregates of antigens, complement proteins, and IgG and IgM antibodies) deposited in various tissues trigger type III hypersensitivity. Type IV hypersensitivity (also known as "delayed type hypersensitivity") usually takes two to three days to develop. Type IV reactions are implicated in many autoimmune and infectious diseases, but also include contact dermatitis. These reactions are mediated by T cells, monocytes, and macrophages.
Other host defense mechanisms
It is likely that the multicomponent, adaptive immune system arose with the earliest vertebrates, since lymphocytes and antibody-based humoral responses are not produced in invertebrates. Many species, however, use mechanisms that appear to be the precursors of these functions of vertebrate immunity. Immune systems appear in even the simplest life forms, such as bacteria, which use a unique defense mechanism called the "restriction and modification system" to ward off viral pathogens called bacteriophages.
Pattern recognition receptors are proteins used by nearly all organisms to identify molecules associated with microbial pathogens. Antimicrobial peptides called defensins constitute a component of the innate immune response that has been conserved throughout evolution, is present in all animals and plants, and represents the major form of systemic immunity in invertebrates. The complement system and phagocytic cells are also present in most invertebrates. Ribonucleases and the RNA interference pathway are conserved in all eukaryotes and are thought to play a role in the immune response to viruses and other foreign genetic material.
Unlike animals, plants do not have phagocytic cells, and the immune response of most plants involves systemic chemical messengers that are distributed throughout the plant. When a part of a plant becomes infected, the The plant generates a localized hypersensitivity response whereby cells at the site of infection undergo rapid apoptosis to prevent the infection from spreading to other parts of the plant. Systemic acquired resistance (SAR) is a type of plant response that renders the entire plant resistant to a particular infectious agent. RNA silencing mechanisms are especially important in this systemic response, as they can block virus replication.
Tumor immunology
Another important function of the immune system is to identify and eliminate tumor cells. Transformed tumor cells express antigens that do not appear on normal cells. The immune system regards these antigens as foreign, causing the immune cells to attack the transformed tumor cells. The antigens expressed by tumors can have various origins; some are derived from oncogenic viruses such as the human papillomavirus, which causes cervical cancer, while others are the body's own proteins that are present at low levels in normal cells, but reach high levels in tumor cells. An example is an enzyme called tyrosinase which, when expressed at high levels, transforms certain skin cells (melanocytes) into tumors called melanomas.
The primary response of the immune system is to destroy abnormal cells by killer T cells, sometimes with the assistance of helper T cells. Tumor antigens are presented bound to MHC class I molecules, similar to what happens with viral antigens. This allows killer T cells to recognize tumor cells as abnormal. Natural killer T cells also kill tumor cells in a similar way, especially if the tumor cell has fewer MHC class I molecules on its surface than normal; something that is common in tumors. Antibodies are sometimes generated against tumor cells, allowing them to be destroyed by the complement system.
However, some tumor cells evade the action of the immune system and cause cancers. One mechanism sometimes used by tumor cells to evade detection by killer T cells is to reduce the number of molecules of the class I MHC on its surface. Some tumor cells also release products that inhibit the immune response, for example by secreting the cytokine TGF-β, which suppresses the activity of macrophages and lymphocytes. In addition, immune tolerance may also develop against to tumor antigens, so that the immune system stops attacking tumor cells.
Physiological regulation
Hormones can modulate the sensitivity of the immune system. For example, female sex hormones are known to stimulate reactions of both the adaptive and innate immune systems. Some autoimmune diseases such as lupus erythematosus more commonly affect females, often coinciding with puberty onset. Conversely, androgens such as testosterone appear to suppress the immune system. Other hormones, such as prolactin and growth hormone, or vitamins such as vitamin D, appear to regulate immune system responses as well. The progressive decline in hormone levels with age may be partly responsible for the weakening of immune responses in elderly individuals. Conversely, some hormones are regulated by the immune system, most notably thyroid hormone activity.
The immune system is boosted by sleep and rest, while it is impaired by stress. Dieting can affect the immune system; for example fresh fruits, vegetables and foods rich in certain fatty acids support the maintenance of a healthy immune system. Also, fetal malnutrition can cause a lifelong weakening of the immune system. In traditional medicines, some plants are believed to They can stimulate the immune system and some studies have suggested this, although their mechanism of action is complex and difficult to characterize.
Sleep and rest
The immune system is affected by sleep and rest, and lack of sleep is detrimental to immune function. Complex feedback loops involving cytokines, such as IL-1 and TNF-α produced in response to infection, also appear to play a role in regulating non-rapid eye movement (REM) sleep. Thus, the immune response to infection can lead to changes in the sleep cycle, including an increase in slow-wave sleep relative to REM sleep.
In people who are sleep deprived, active immunizations may have a diminished effect and may result in less antibody production and a lower immune response than would be seen in a well-rested person. In addition, proteins such as NFIL3, which have been shown to be closely intertwined with both T cell differentiation and circadian rhythms, may be affected by disruption of natural light and dark cycles through sleep deprivation.. These disturbances can lead to an increase in chronic conditions such as heart disease, chronic pain, and asthma.
In addition to the negative consequences of sleep deprivation, sleep and the intertwined circadian system have been shown to have strong regulatory effects on immune functions affecting both innate and adaptive immunity. First, during early slow-wave sleep, a sudden drop in blood levels of cortisol, epinephrine, and norepinephrine causes blood levels of the hormones leptin, pituitary growth hormone, and prolactin to rise. These signals induce a proinflammatory state through the production of the proinflammatory cytokines IL-1, IL-12, TNF-alpha, and interferon gamma. These cytokines then stimulate immune functions such as activation, proliferation, and differentiation of immune cells. During this time of a slowly evolving adaptive immune response, there is a spike in undifferentiated or less differentiated cells, such as central and naïve memory T cells. In addition to these effects, the milieu of hormones produced at this time (leptin, pituitary growth hormone, and prolactin) support interactions between antigen presenting cells (APCs) and T cells, a shift in the balance of Th1/Th2 cytokines. toward one that supports Th1, an increase in overall Th cell proliferation and migration of naïve T cells to lymph nodes. This is also believed to promote the formation of a durable immune memory by initiating Th1 immune responses.
During periods of wakefulness, differentiated effector cells, such as NK cells and cytotoxic T lymphocytes, peak to elicit an effective response against any intruding pathogens. Anti-inflammatory molecules, such as cortisol and catecholamines, also peak during active times of wakefulness. Inflammation would cause severe cognitive and physical impairments if it occurred during waking hours, and inflammation can occur during sleeping hours due to the presence of melatonin. Inflammation causes a great deal of oxidative stress, and the presence of melatonin during sleep hours could actively counteract free radical production during this time.
Manipulation in medicine
The immune response can be manipulated to suppress unwanted autoimmune responses, allergy and transplant rejection, as well as stimulate protective responses against pathogens that largely evade the action of the immune system. Immunosuppressive drugs are used to control autoimmune diseases or inflammation when it causes extensive tissue damage, or to prevent rejection of a transplanted organ.
Anti-inflammatory drugs are used to control the effects of inflammation. Corticosteroids are the most powerful of these drugs; however, they have many toxic side effects and their use must be strictly controlled. Therefore, lower doses of anti-inflammatories are often used in conjunction with immunosuppressive and cytotoxic drugs such as methotrexate or azathioprine. Cytotoxic drugs inhibit the immune response by destroying cells that are dividing, such as T cells that have been activated. However, the destruction is indiscriminate, so other organs and cell types are affected, resulting in side effects. Immunosuppressive drugs such as cyclosporine prevent T cells from responding correctly to signals, inhibiting signal transduction pathways..
Drugs with higher molecular weight (>500 daltons) can cause neutralization of the immune response, particularly if they are administered repeatedly, or in large doses. This limits the efficacy of drugs made up of large peptides and proteins (generally exceeding 6000 daltons). In some cases, the drug is not immunogenic itself, but may be co-administered with an immunogenic drug, such as Taxol. Computational methods have been developed to predict the immunogenicity of peptides and proteins, which are particularly useful in the design of therapeutic antibodies, the assessment of the likely virulence of mutations affecting viral coat particles, and the validation of new peptide-based drugs.. The first techniques were based mainly on the observed fact that hydrophilic amino acids are present, in greater quantity than hydrophobic amino acids, in epitopes (antigenic determinants that produce a reversible specific interaction with an immunoglobulin and consist of a group of localized amino acids). on the surface of the antigen); however, more recently Machine Learning techniques have been used, which use databases of known epitopes, usually well-studied viral proteins. A publicly accessible database has been created for cataloging epitopes of pathogens known to be recognized by B cells. Bioinformatics-based immunogenicity studies are an emerging field known as immunoinformatics.
Handling by pathogens
The success of any pathogen depends on its ability to evade the host's immune responses. Therefore, pathogens have evolved different methods that allow them to successfully infect the host, while evading the destruction produced by immunity. Bacteria often manage to bypass physical barriers by secreting enzymes that digest the barrier—for example, using a type II secretion system. Alternatively, using a type III secretion system, they can insert a hollow tube into the host cell that provides them with a conduit for transporting proteins from pathogen to host; the proteins carried by the tube are frequently used to disarm the host's defenses.
One strategy used by various pathogens to evade the innate immune system is intracellular replication (also called intracellular pathogenesis). In it, a pathogen spends most of its life cycle inside host cells where it is protected from direct contact with immune cells, antibodies, and complement proteins. Some examples of intracellular pathogens include viruses, bacteria of the genus Salmonella that cause food poisoning, and the eukaryotic parasites that cause malaria (Plasmodium falciparum) and leishmaniasis (Leishmania spp.). Other bacteria, such as Mycobacterium tuberculosis, live within a protective capsule that prevents lysis by complement. Many pathogens secrete compounds that diminish or divert the host's immune response. Some bacteria form biofilms to protect themselves from the cells and proteins of the immune system. These biofilms are present in many successful infections, such as chronic infections caused by Pseudomonas aeruginosa and Burkholderia cenocepacia characteristic of Cystic Fibrosis. Other bacteria generate proteins surface cells that bind to antibodies, rendering them ineffective. Examples include: streptococci (G protein), Staphylococcus aureus (A protein), and Peptostreptococcus magnus (L protein).
The mechanisms used by viruses to evade the adaptive immune system are more complex. The simplest approach is to rapidly change the non-essential epitopes (amino acids or sugars) on the invader's surface, while keeping the essential epitopes hidden. HIV, for example, regularly mutates proteins in its viral envelope that are essential for it to enter its target host cells. These frequent changes in antigens may explain the failure to produce vaccines directed against these proteins. Another common strategy to avoid detection by the immune system is to mask their antigens with host cell proteins. Thus, in HIV, the envelope that covers the virion is formed by the outermost membrane of the host cell; such "self-cloaking" viruses make it difficult for the immune system to identify them as not self.
History of Immunology
Immunology is a science that examines the structure and function of the immune system. It originates from medicine and early studies on the causes of immunity to disease. The earliest reference to immunity occurs during the plague of Athens in 430 BC. C., where Thucydides noted that some people who had recovered from a previous outbreak of the disease could care for the sick without contracting the disease a second time. This observation of acquired immunity was later used by Louis Pasteur in the development of the vaccination and in his microbial theory of disease. Pasteur's theory was in opposition to contemporary theories of disease, such as the miasmatic theory. Microorganisms were not confirmed as the cause of infectious diseases until 1891, when Robert Koch enunciated his postulates, for which he received the Nobel Prize in 1905. In 1901, with the discovery of the yellow fever virus by Walter Reed, the viruses were confirmed to be human pathogens.
A breakthrough in immunology occurred towards the end of the 19th century, thanks to the rapid development of immunology studies. humoral immunity and cellular immunity. Of particular importance was the work of Paul Ehrlich, who proposed the Side Chain Theory to explain the specificity of the antigen-antibody reaction; His contributions to the understanding of humoral immunology were recognized with the Nobel Prize in 1908, received jointly with Elie Metchnikoff, the founder of cellular immunology.
Peter Gorer discovered the mouse H-2 antigen in 1936, and produced the first major histocompatibility complex (MHC). Meanwhile, Peter Medawar and Thomas Gibson may shed light on important functions of immune cells. In 1948, Astrid Fagraeus discovered that antibodies are produced by B lymphocytes in the plasma. A year later, Frank Macfarlane Burnet and Frank Fenner published their hypothesis on immune tolerance, which would be confirmed a few years later by Jacques Miller with the discovery of the elimination of autoreactive T lymphocytes in the thymus. In 1957, Frank Macfarlane Burnet described the theory of clonal selection as the central tenet of adaptive immunity.
In the late 1960s and early 1970s, John David and Barry Bloom discovered Macrophage Migration Inhibitory Factor (MIF) and a new class of substances secreted by lymphocytes. Dudley Dumonde coined the term "lymphokine" for these substances. Stanley Cohen, who in 1986 won the Nobel Prize in Physiology or Medicine for his discovery of the growth factors NGF and EGF, began to study in the early 1970s the functions of factors called "lymphokines" 3. 4; along with Takeshi Yoshida. They discovered that these substances belong to a group of messenger substances that are produced by many different types of cells of the immune system. In 1974 Stanley Cohen proposed the term "cytokine", which was consolidated with the discovery of more substances of this type. Since then more than a hundred new cytokines have been discovered, the structure and functions of which have been investigated in detail.
Contenido relacionado
Magnoliaceae
Erianthus
Human anatomy